On Fahrenheit Scale Water Boils At
News Leon
Mar 18, 2025 · 6 min read
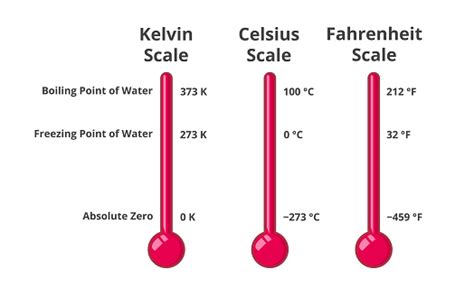
Table of Contents
On the Fahrenheit Scale, Water Boils At… 212°F: A Deep Dive into Temperature Measurement
The seemingly simple question, "On the Fahrenheit scale, water boils at...?" holds a surprising depth of scientific and historical context. The answer, of course, is 212°F, but understanding why this is the case requires exploring the fascinating world of thermometry, the history of temperature scales, and the properties of water itself. This comprehensive guide delves into these aspects, offering a nuanced perspective beyond the simple numerical answer.
Understanding the Fahrenheit Scale
The Fahrenheit scale, named after the German-Dutch physicist Daniel Gabriel Fahrenheit, is one of the most commonly used temperature scales globally, despite the metric system's prevalence in scientific circles. While the Celsius (or Centigrade) scale is favored for its scientific simplicity and logical structure (0°C for freezing water and 100°C for boiling water at standard atmospheric pressure), Fahrenheit remains ingrained in everyday life in the United States and a few other countries.
The Origins of Fahrenheit's Scale
Fahrenheit's scale wasn't arbitrarily defined. His initial zero point, 0°F, was based on a specific reproducible temperature: a mixture of ice, water, and ammonium chloride. This "freezing mixture" created a remarkably stable low temperature that served as his reference point. His upper limit was initially based on human body temperature, initially set at 96°F (later refined), reflecting the importance of human physiology at the time.
This choice, while now seeming arbitrary, reflected the state of scientific knowledge and practical needs in the early 18th century. While it may seem less intuitive than Celsius, it provides sufficient granularity for practical applications.
Why 212°F for Boiling Water?
The boiling point of water at standard atmospheric pressure (1 atmosphere, or 101.325 kPa) is the key to understanding why 212°F is crucial on the Fahrenheit scale. This point, 212°F, wasn't arbitrarily assigned. It's the result of Fahrenheit's experimental observations and the calibration of his scale. He carefully measured the boiling point of water under standard conditions, establishing this benchmark for his scale. This landmark temperature became an integral part of the Fahrenheit scale's definition and remains a widely recognized reference point.
The Importance of Standard Atmospheric Pressure
It's crucial to emphasize that the boiling point of water is dependent on atmospheric pressure. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, such as in a pressure cooker, water boils at a higher temperature. The 212°F boiling point specifically applies to water boiling under standard atmospheric pressure at sea level. Any deviation from this standard pressure will result in a different boiling point.
The Physics of Boiling: A Molecular Perspective
To fully appreciate the significance of 212°F, we must delve into the physics of boiling. Boiling is a phase transition, a change in the physical state of matter from liquid to gas. At the molecular level, it involves:
Molecular Kinetic Energy and Vapor Pressure
Water molecules are constantly in motion, possessing kinetic energy. As the temperature of water increases, the average kinetic energy of its molecules also increases. This increased kinetic energy allows more molecules to overcome the intermolecular forces holding them together in the liquid phase. These molecules escape the liquid's surface, forming water vapor.
The pressure exerted by this escaping vapor is called vapor pressure. As the temperature rises, the vapor pressure increases. When the vapor pressure of the liquid equals the external pressure (atmospheric pressure), boiling occurs. At 212°F and standard atmospheric pressure, the vapor pressure of water is exactly 1 atmosphere, leading to vigorous boiling.
Nucleation Sites and Bubble Formation
Boiling isn't a uniform process across the entire liquid volume. It typically starts at nucleation sites – microscopic imperfections on the surface of the container or tiny air bubbles trapped within the liquid. These sites provide surfaces for vapor bubbles to form and grow. As more molecules transition from liquid to gas, these bubbles expand and eventually rise to the surface, releasing the vapor into the air.
Heat Transfer and Latent Heat of Vaporization
Boiling requires continuous energy input in the form of heat. This heat energy isn't used to increase the temperature of the water but instead to overcome the intermolecular forces holding the liquid together. This energy is called the latent heat of vaporization. It's the energy required to change one gram of water from liquid to gas at its boiling point. This is a significant amount of energy, contributing to the cooling effect of evaporation.
The Relationship Between Fahrenheit, Celsius, and Kelvin
The Fahrenheit scale, while widely used, isn't the preferred scale in scientific contexts. The Celsius scale, with its 0°C freezing point and 100°C boiling point for water, offers a more logical and intuitive structure. The Kelvin scale, the absolute temperature scale, offers even greater scientific benefits.
Converting Between Scales
The conversions between these scales are straightforward:
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
- Fahrenheit to Celsius: °C = (°F - 32) × 5/9
- Celsius to Kelvin: K = °C + 273.15
- Fahrenheit to Kelvin: K = (°F - 32) × 5/9 + 273.15
Understanding these conversions is crucial for comparing temperature measurements across different scales and for appreciating the relationships between the various temperature scales. 212°F corresponds to 100°C and 373.15 K.
Applications and Importance of the Boiling Point of Water
The boiling point of water, whether expressed in Fahrenheit, Celsius, or Kelvin, is fundamental to countless applications in various fields:
Cooking and Food Preparation
The boiling point of water is a critical parameter in cooking, affecting cooking times and food textures. Understanding that water boils at a lower temperature at high altitudes requires adjusting cooking methods to achieve desired results.
Industrial Processes
Numerous industrial processes rely on the boiling point of water for sterilization, cleaning, and various chemical reactions. From power generation to food processing, the boiling point serves as a crucial reference point.
Scientific Experiments and Research
The precise boiling point of water under standard conditions is a fundamental constant in scientific measurements and calibrations. It serves as a benchmark for thermometry and heat transfer studies.
Meteorology and Climate Science
Water's boiling point plays a critical role in understanding atmospheric processes, evaporation rates, and climate models. Variations in boiling point due to pressure changes are important considerations in meteorological studies.
Conclusion: More Than Just a Number
The seemingly simple question of water's boiling point on the Fahrenheit scale – 212°F – opens a window into a rich tapestry of scientific concepts, historical developments, and practical applications. From the historical context of Fahrenheit's scale to the underlying physics of boiling and phase transitions, understanding this point provides valuable insights into the world around us. Its importance extends far beyond a simple numerical value, serving as a cornerstone in various scientific disciplines and everyday life. By appreciating the significance of 212°F, we gain a deeper understanding of temperature measurement, the properties of water, and the interconnectedness of science and our daily experiences.
Latest Posts
Related Post
Thank you for visiting our website which covers about On Fahrenheit Scale Water Boils At . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.