Number Of Valence Electrons In Lithium
News Leon
Mar 18, 2025 · 6 min read
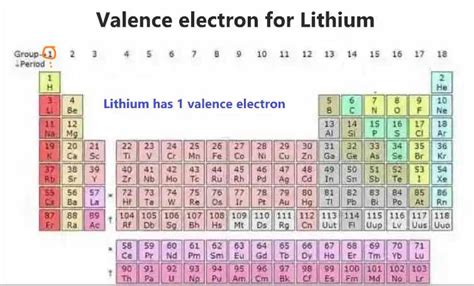
Table of Contents
Delving Deep into Lithium: Unveiling the Secrets of its Valence Electrons
Lithium, the lightest of the alkali metals, holds a unique position in the periodic table and in the world of chemistry. Its properties, reactivity, and applications are all intricately linked to a single, crucial factor: its number of valence electrons. This article delves deep into the fascinating world of lithium, exploring its electronic structure, the significance of its single valence electron, and the implications this has on its chemical behavior and diverse applications.
Understanding Valence Electrons: The Key to Reactivity
Before we dive into the specifics of lithium, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most likely to participate in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons dictates an element's position in the periodic table and its chemical behavior. Elements within the same group (vertical column) possess the same number of valence electrons, explaining their similar chemical properties.
Lithium's Electronic Configuration: A Single Electron Makes All the Difference
Lithium (Li), with an atomic number of 3, possesses three electrons in total. Its electronic configuration is 1s²2s¹. This configuration reveals the distribution of electrons across different energy levels or shells. The '1s²' signifies two electrons occupying the first energy level (or shell), while '2s¹' indicates a single electron in the second energy level. Crucially, this single electron in the 2s orbital is lithium's valence electron.
The Significance of the Single Valence Electron
This solitary valence electron is the key to understanding lithium's properties and reactivity. Atoms strive for stability, typically achieved by having a full outer electron shell. Lithium, with only one electron in its valence shell, readily loses this electron to attain a stable configuration resembling that of helium (1s²), a noble gas with a completely filled outer shell. This process, known as ionization, makes lithium highly reactive.
Lithium's Reactivity: A Consequence of its Valence Electron
The ease with which lithium loses its valence electron makes it highly reactive, particularly with electronegative elements like halogens (Group 17) and oxygen. This reactivity is evident in various chemical reactions:
Reactions with Halogens: Formation of Ionic Compounds
Lithium readily reacts with halogens (fluorine, chlorine, bromine, iodine) to form ionic compounds known as lithium halides (LiF, LiCl, LiBr, LiI). In these reactions, lithium loses its valence electron to the halogen atom, forming a positively charged lithium ion (Li⁺) and a negatively charged halide ion (F⁻, Cl⁻, Br⁻, I⁻). The electrostatic attraction between these oppositely charged ions leads to the formation of a stable ionic crystal lattice.
Reactions with Oxygen: Formation of Lithium Oxide
Lithium reacts vigorously with oxygen (O₂) in the air to form lithium oxide (Li₂O). Again, this reaction involves the loss of lithium's valence electron. Two lithium atoms each lose one electron to an oxygen atom, which gains two electrons to achieve a stable octet. The resulting Li⁺ ions and O²⁻ ions are held together by strong electrostatic forces.
Reactions with Water: A Vigorous Reaction
Lithium reacts violently with water, producing hydrogen gas (H₂) and lithium hydroxide (LiOH). This exothermic reaction generates considerable heat, further emphasizing lithium's high reactivity due to its single valence electron. The reaction involves the transfer of lithium's valence electron to a hydrogen atom in a water molecule, resulting in the formation of hydrogen gas and the hydroxide ion.
Applications of Lithium: Leveraging its Unique Properties
Lithium's unique properties stemming from its single valence electron have led to numerous applications across diverse fields:
Lithium-ion Batteries: Powering Portable Electronics and Electric Vehicles
One of the most significant applications of lithium is in lithium-ion batteries. These batteries utilize lithium ions (Li⁺) to facilitate the flow of electrical charge between the anode and cathode. The ease with which lithium ions can lose and gain electrons, coupled with their small size and high mobility, makes them ideal for use in high-energy-density batteries. Lithium-ion batteries power a wide range of devices, from smartphones and laptops to electric vehicles and grid-scale energy storage systems. The demand for lithium-ion batteries continues to grow exponentially, driving the exploration and exploitation of lithium resources worldwide.
Lubricants and Greases: Enhancing Performance and Durability
Lithium's ability to form stable compounds with various organic molecules has made it a valuable component in high-performance lubricants and greases. Lithium-based greases exhibit excellent high-temperature stability, water resistance, and load-bearing capacity, making them suitable for applications in automotive, industrial, and aerospace settings.
Ceramics and Glass: Improving Strength and Durability
Lithium compounds are also used in the production of ceramics and glasses. The addition of lithium oxide (Li₂O) to glass improves its durability, strength, and chemical resistance. Lithium-containing ceramics exhibit enhanced thermal shock resistance and mechanical strength, making them suitable for high-temperature applications.
Medical Applications: Treating Bipolar Disorder and Other Conditions
Lithium salts, specifically lithium carbonate (Li₂CO₃), have found application in the treatment of bipolar disorder and other mental health conditions. While the exact mechanism of action is not fully understood, lithium ions are believed to influence neurotransmitter levels in the brain, helping to stabilize mood and reduce the severity of mood swings.
Exploring Lithium's Isotopes: Variations in Neutron Number
While all lithium atoms possess three protons and three electrons, they can exhibit variations in their neutron number. These variations are known as isotopes. The two most abundant isotopes are lithium-6 (⁶Li) and lithium-7 (⁷Li). ⁶Li has three protons and three neutrons, while ⁷Li has three protons and four neutrons. The difference in neutron number does not significantly affect the chemical properties of lithium, as the chemical behavior is predominantly determined by the number of electrons (and hence the valence electrons). However, the different isotopes exhibit variations in their nuclear properties, influencing their applications in nuclear physics and other specialized fields.
Conclusion: The Enduring Significance of Lithium's Single Valence Electron
Lithium's single valence electron is the cornerstone of its unique properties and wide-ranging applications. This seemingly simple feature governs its high reactivity, its ability to form ionic compounds, and its suitability for diverse technological and medical applications. From powering our portable electronics and electric vehicles to stabilizing mood and enhancing the durability of ceramics, lithium's influence is pervasive. Understanding the behavior of its valence electron remains crucial for further advancements in these and other areas, highlighting the importance of fundamental chemical principles in shaping our technological landscape and improving human life. The continued research and development focused on lithium will undoubtedly unlock further applications and refine existing technologies, solidifying its position as a critical element for the future.
Latest Posts
Latest Posts
-
Receptacle Is Part Of The Four Whorls
Mar 19, 2025
-
The Bending Of Waves Around A Barrier
Mar 19, 2025
-
A Surveyor Measures The Distance Across A Straight River
Mar 19, 2025
-
Fluid Pressure Against A Wall Or Cell Membranes Is Called
Mar 19, 2025
-
How Many Micrograms In A Kilogram
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
