Number Of Valence Electrons In Li
News Leon
Mar 23, 2025 · 5 min read
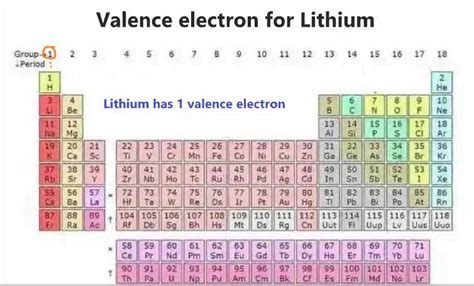
Table of Contents
Unveiling the Secrets of Lithium's Valence Electrons: A Deep Dive
Lithium (Li), the lightest alkali metal, holds a unique position in the periodic table. Understanding its electronic configuration, particularly the number of valence electrons, is crucial to grasping its remarkable reactivity and diverse applications. This comprehensive article will delve into the intricacies of lithium's valence electrons, exploring its electronic structure, chemical bonding, and the implications for its properties and uses.
Understanding Valence Electrons: The Key to Reactivity
Before we focus specifically on lithium, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they are the ones involved in chemical bonding with other atoms. The number of valence electrons directly determines an element's reactivity and the types of chemical bonds it can form. Elements strive to achieve a stable electron configuration, often resembling the nearest noble gas, through gaining, losing, or sharing valence electrons. This pursuit of stability is the driving force behind chemical reactions.
The Significance of the Octet Rule
The octet rule is a fundamental principle in chemistry stating that atoms tend to gain, lose, or share electrons in order to have eight electrons in their outermost shell, achieving the stable electron configuration of a noble gas. While there are exceptions, this rule provides a valuable framework for predicting the behavior of many elements, including lithium. However, it's important to note that the octet rule doesn't always apply perfectly, particularly for elements with low atomic numbers like lithium.
Delving into Lithium's Electronic Structure: The "2,1" Configuration
Lithium, with an atomic number of 3, possesses three electrons in total. Its electronic configuration is represented as 1s²2s¹. This notation indicates that two electrons occupy the first energy level (1s orbital), and one electron resides in the second energy level (2s orbital). It's the electron in the 2s orbital that is the key player – it's lithium's sole valence electron.
Visualizing Lithium's Electron Arrangement
Imagine the nucleus of the lithium atom at the center, with its three protons. The two electrons in the 1s orbital are closest to the nucleus, forming a relatively stable inner shell. The lone electron in the 2s orbital is further away, loosely held and readily available for interaction with other atoms. This solitary valence electron is the reason behind lithium's high reactivity.
The Role of the Valence Electron in Lithium's Reactivity
Because lithium has only one valence electron, it readily loses this electron to achieve a stable configuration resembling helium (He), a noble gas with a filled 1s² shell. This electron loss results in the formation of a positively charged lithium ion (Li⁺). This tendency to lose an electron explains lithium's strong tendency to participate in ionic bonding.
Ionic Bonding: Lithium's Preferred Method
Ionic bonding occurs when one atom loses an electron (becoming a cation) and another atom gains an electron (becoming an anion). The resulting ions are held together by electrostatic forces of attraction. Lithium, with its readily available valence electron, readily forms ionic bonds with electronegative elements, such as halogens (fluorine, chlorine, bromine, iodine) and oxygen.
Examples of Ionic Compounds with Lithium:
- Lithium fluoride (LiF): Lithium loses its valence electron to fluorine, forming Li⁺ and F⁻ ions.
- Lithium oxide (Li₂O): Two lithium atoms each lose one electron to an oxygen atom, forming 2Li⁺ and O²⁻ ions.
- Lithium chloride (LiCl): Lithium loses its valence electron to chlorine, forming Li⁺ and Cl⁻ ions.
These ionic compounds are typically crystalline solids with high melting points due to the strong electrostatic forces between the oppositely charged ions.
Lithium's Properties and Their Connection to Valence Electrons
The single valence electron in lithium dictates many of its characteristic properties:
- Low Ionization Energy: Lithium has a relatively low ionization energy, meaning it requires relatively little energy to remove its valence electron. This directly stems from the fact that the single valence electron is shielded from the nuclear charge by the inner shell electrons, making it easier to remove.
- Low Electronegativity: Lithium has a low electronegativity, meaning it has a low tendency to attract electrons towards itself. This is consistent with its tendency to lose its valence electron rather than gain one.
- Metallic Character: Lithium exhibits metallic characteristics, such as good electrical and thermal conductivity. These properties are attributed to the delocalized valence electrons, which can move freely throughout the metal lattice.
- Reactivity: As previously discussed, lithium's single valence electron makes it highly reactive, particularly with water and other electronegative elements. This reactivity is a defining characteristic of alkali metals.
Applications of Lithium: From Batteries to Medicine
The unique properties of lithium, stemming directly from its single valence electron, lead to its diverse applications in various fields:
-
Lithium-ion Batteries: Lithium-ion batteries are ubiquitous in portable electronic devices, electric vehicles, and energy storage systems. Lithium's ability to readily lose and gain electrons, coupled with its relatively low atomic weight, makes it an ideal component in these rechargeable batteries. The battery's performance is directly linked to lithium's electronic properties.
-
Lubricants: Lithium-based greases are used as high-temperature lubricants due to their thermal stability and resistance to oxidation. These properties are influenced by lithium's chemical bonding and interaction with other molecules.
-
Ceramics and Glass: Lithium compounds are used in the production of specialized ceramics and glasses due to their impact on the material's properties, such as thermal expansion and strength.
-
Medicines: Lithium salts have been used in medicine for the treatment of bipolar disorder. The mechanism of action isn't fully understood, but it's believed to involve interactions with ion channels and neurotransmitters within the nervous system.
Conclusion: The Importance of One Electron
Lithium's single valence electron is not just a numerical detail; it's the key to understanding its chemical behavior, properties, and diverse applications. From its highly reactive nature to its role in advanced technologies, the behavior of this single electron dictates the remarkable impact lithium has on our modern world. The ability to predict and manipulate the behavior of valence electrons is a cornerstone of chemistry and materials science, highlighting the significance of understanding fundamental atomic structure. Further research into lithium's intricate electronic interactions promises even more innovative applications in the future. The simple elegance of its atomic configuration belies the immense complexity and importance of this element in the scientific and technological landscape.
Latest Posts
Latest Posts
-
Animals That Can Grow Lungs After Being Born
Mar 25, 2025
-
The Ability To Respond To A Stimulus
Mar 25, 2025
-
What Is The Area Of Triangle Lmn
Mar 25, 2025
-
The Figure Shows A Circular Region Of Radius R
Mar 25, 2025
-
30 Is 60 Percent Of What Number
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Li . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
