Number Of Valence Electrons In Br
News Leon
Mar 18, 2025 · 5 min read
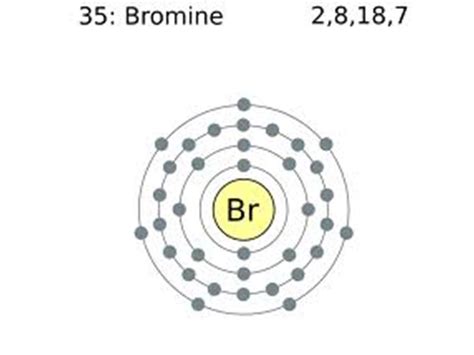
Table of Contents
Delving Deep into the Valence Electrons of Bromine (Br)
Bromine (Br), a vibrant reddish-brown liquid element, holds a fascinating position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and reactivity. This comprehensive article will explore the intricacies of bromine's valence electrons, covering its atomic structure, its position within the periodic table, its bonding characteristics, and its implications in various chemical reactions.
Understanding Valence Electrons: The Key to Reactivity
Before diving into the specifics of bromine, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary players in chemical bonding, determining an atom's reactivity and the types of bonds it can form. They dictate how an atom will interact with other atoms, influencing the formation of molecules and compounds. Atoms strive for stability, often achieved by having a full outermost electron shell (an octet, typically eight electrons, though there are exceptions). This drive for stability governs the chemical behavior of elements.
Bromine's Position in the Periodic Table: A Clue to its Valence Electrons
Bromine's location in the periodic table provides a crucial shortcut to determining its number of valence electrons. Bromine (Br) is a halogen, belonging to Group 17 (or VIIA) of the periodic table. Elements within the same group share similar properties, primarily due to having the same number of valence electrons.
Group 17 elements, the halogens, are characterized by having seven valence electrons. This common characteristic dictates their strong tendency to gain one electron to achieve a stable octet configuration, making them highly reactive and prone to forming -1 anions.
Bromine's Electronic Configuration: A Detailed Look
To fully grasp the concept of bromine's valence electrons, we need to examine its electronic configuration. The electronic configuration describes how electrons are distributed among the various energy levels (shells) and sublevels within an atom. Bromine's atomic number is 35, indicating it possesses 35 electrons. Its electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the filled inner shells, also known as core electrons. These electrons are not involved in chemical bonding.
-
4s² 3d¹⁰ 4p⁵: These are the electrons in the outermost shell, also known as the valence shell. This is where the magic happens!
Adding up the electrons in the outermost shell (4s² + 4p⁵), we find that bromine has seven valence electrons. This confirms its placement in Group 17 and explains its high reactivity.
Bromine's Chemical Bonding: A Consequence of its Valence Electrons
The seven valence electrons of bromine directly influence its chemical behavior and bonding patterns. Because it's one electron short of a stable octet, bromine readily forms ionic bonds by gaining one electron from another atom, thereby acquiring a -1 charge and forming a bromide ion (Br⁻).
This is clearly seen in the formation of ionic compounds such as sodium bromide (NaBr), where sodium (Na), with one valence electron, readily donates it to bromine, forming Na⁺ and Br⁻ ions, which are electrostatically attracted to each other.
Bromine also readily forms covalent bonds, sharing its electrons with other atoms to complete its octet. For example, in diatomic bromine (Br₂), two bromine atoms share one pair of electrons, achieving a stable configuration for both atoms. This covalent bond results in the characteristic reddish-brown liquid form of bromine at room temperature.
Applications of Bromine and the Role of its Valence Electrons
Bromine's unique properties, stemming directly from its seven valence electrons, lead to a wide range of applications across various industries. These include:
-
Flame Retardants: Bromine-containing compounds are used extensively as flame retardants in plastics, textiles, and electronics. The ability of bromine to form strong bonds helps disrupt the combustion process.
-
Agricultural Chemicals: Bromine compounds are used as fumigants and pesticides to control pests and diseases in agriculture.
-
Water Treatment: Bromine compounds can act as disinfectants, purifying water by killing harmful bacteria and microorganisms.
-
Pharmaceuticals: Bromine compounds are found in certain pharmaceutical products, serving various roles depending on the specific compound.
-
Photography: Historically, silver bromide (AgBr) played a crucial role in photographic film, enabling light-sensitive reactions.
Exceptions and Considerations
While the octet rule is a useful guideline, it's important to remember that there are exceptions. Some compounds involve bromine with fewer or more than eight electrons surrounding it. These exceptions often involve transition metals or elements in the later periods of the periodic table. The expansion or contraction of the valence shell arises from the availability of d-orbitals that can participate in bonding.
Conclusion: The Significance of Bromine's Valence Electrons
The number of valence electrons in an atom profoundly impacts its chemical properties and reactivity. Bromine, with its seven valence electrons, exhibits a strong tendency to gain one electron to achieve a stable octet. This characteristic leads to its high reactivity, its ability to form ionic and covalent bonds, and consequently, its diverse range of applications in various industries. Understanding the electronic configuration and the implications of valence electrons is key to comprehending the chemical behavior of bromine and its importance in the world around us. Further exploration into the specific applications of bromine and its compounds would uncover even more profound details about the role of these seven electrons in shaping the element's properties and function. The significance of these valence electrons extends beyond simple chemical reactions, influencing macroscopic properties and technological applications. Continual research into bromine chemistry ensures a deeper understanding of this vital element and its importance in our world. The detailed investigation into its bonding characteristics and reactions provides valuable insight into broader chemical principles and applications.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Br . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
