Number Of Valence Electrons In As
News Leon
Apr 07, 2025 · 7 min read
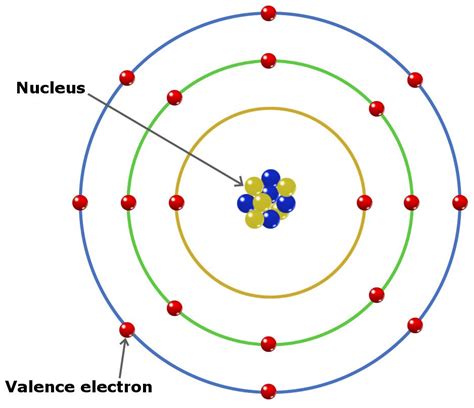
Table of Contents
Number of Valence Electrons in Atoms: A Comprehensive Guide
Determining the number of valence electrons in an atom is crucial for understanding its chemical behavior and reactivity. Valence electrons are the electrons located in the outermost shell of an atom, and they are directly involved in chemical bonding. This comprehensive guide will delve into the methods for determining the number of valence electrons, focusing on various elements and providing a thorough understanding of this fundamental concept in chemistry.
Understanding Valence Electrons and Their Significance
Valence electrons are the electrons residing in the outermost energy level or shell of an atom. These electrons are the most loosely held and therefore participate in chemical reactions, forming bonds with other atoms. The number of valence electrons dictates an element's reactivity and the types of bonds it can form (ionic, covalent, or metallic). Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outermost shell (usually eight electrons, following the octet rule, except for hydrogen and helium, which aim for two electrons).
Understanding valence electrons is fundamental to:
- Predicting chemical bonding: The number of valence electrons dictates how an atom will bond with other atoms. Atoms tend to react in ways that result in a full outer electron shell.
- Determining the oxidation state: The oxidation state of an element reflects the number of electrons it has gained or lost in a chemical reaction, and this is directly related to its valence electrons.
- Explaining periodic trends: Valence electron configurations explain many periodic trends, such as electronegativity, ionization energy, and atomic radius.
Methods for Determining the Number of Valence Electrons
There are several ways to determine the number of valence electrons in an atom:
1. Using the Periodic Table: The Easiest Method
The most straightforward method utilizes the periodic table. The group number (vertical column) of an element in the periodic table (using the American system of numbering groups 1-18) often directly corresponds to the number of valence electrons for many main group elements.
- Groups 1 and 2 (Alkali and Alkaline Earth Metals): Elements in these groups have 1 and 2 valence electrons, respectively.
- Groups 13-18: Elements in these groups generally have 3, 4, 5, 6, 7, and 8 valence electrons, respectively. However, there are exceptions, particularly for transition metals (d-block elements) and inner transition metals (f-block elements).
Important Note: This method is simplified and doesn't apply to transition metals and inner transition metals. Their valence electron configuration is more complex due to the involvement of d and f orbitals.
2. Using Electron Configuration: A Precise Method
Electron configuration precisely describes the arrangement of electrons in an atom's orbitals. It provides a definitive way to identify the number of valence electrons. The electron configuration is written using a notation that indicates the principal energy level (n), the type of orbital (s, p, d, f), and the number of electrons in each orbital.
For example, the electron configuration of oxygen (O) is 1s²2s²2p⁴. The valence electrons are those in the highest principal energy level (n=2 in this case). Therefore, oxygen has 6 valence electrons (2s²2p⁴).
To determine the number of valence electrons using this method:
- Write the element's electron configuration: You can either memorize or look up the electron configuration.
- Identify the highest principal energy level: This is the outermost shell.
- Count the electrons in the highest energy level: This number represents the number of valence electrons.
Example: Let's find the number of valence electrons in phosphorus (P).
The electron configuration of phosphorus is 1s²2s²2p⁶3s²3p³. The highest energy level is n=3, and it contains 5 electrons (3s²3p³). Therefore, phosphorus has 5 valence electrons.
3. Using the Group Number (Alternative Method): Addressing Exceptions
While the group number method is generally reliable for main group elements, exceptions exist, especially for transition metals and inner transition metals. These elements have incompletely filled d or f orbitals, which complicate the valence electron count. Their variable oxidation states reflect the varying numbers of valence electrons they can contribute in chemical reactions.
For transition metals, the number of valence electrons isn't always directly determined by the group number. They can use electrons from both the s and d orbitals as valence electrons, leading to multiple possible oxidation states. For example, iron (Fe) can have +2 or +3 oxidation states, indicating its variability in valence electron participation.
Inner transition metals (lanthanides and actinides) exhibit even greater complexity in their electron configurations and valence electron counts due to the filling of the f orbitals.
Valence Electrons and Chemical Bonding
The number of valence electrons is directly linked to the type of chemical bonds an atom will form:
-
Ionic Bonds: Formed when one atom loses valence electrons to another atom, resulting in the formation of ions (cations and anions). This commonly occurs between metals (which tend to lose electrons) and nonmetals (which tend to gain electrons). For example, the reaction between sodium (1 valence electron) and chlorine (7 valence electrons) results in the formation of sodium chloride (NaCl), an ionic compound.
-
Covalent Bonds: Formed when atoms share valence electrons to achieve a stable electron configuration. This typically occurs between nonmetals. For example, the two oxygen atoms in an oxygen molecule (O₂) share electrons to form a double covalent bond.
-
Metallic Bonds: Occur in metals, where valence electrons are delocalized and shared among a large number of atoms. This creates a "sea" of electrons that holds the metal atoms together.
Illustrative Examples Across the Periodic Table
Let's explore the number of valence electrons for selected elements across the periodic table:
Group 1 (Alkali Metals):
- Lithium (Li): Electron configuration: 1s²2s¹; Valence electrons: 1
- Sodium (Na): Electron configuration: 1s²2s²2p⁶3s¹; Valence electrons: 1
- Potassium (K): Electron configuration: 1s²2s²2p⁶3s²3p⁶4s¹; Valence electrons: 1
All alkali metals readily lose their single valence electron to form +1 ions.
Group 17 (Halogens):
- Fluorine (F): Electron configuration: 1s²2s²2p⁵; Valence electrons: 7
- Chlorine (Cl): Electron configuration: 1s²2s²2p⁶3s²3p⁵; Valence electrons: 7
- Bromine (Br): Electron configuration: [Ar]3d¹⁰4s²4p⁵; Valence electrons: 7
Halogens readily gain one electron to achieve a stable octet, forming -1 ions.
Group 18 (Noble Gases):
- Helium (He): Electron configuration: 1s²; Valence electrons: 2
- Neon (Ne): Electron configuration: 1s²2s²2p⁶; Valence electrons: 8
- Argon (Ar): Electron configuration: 1s²2s²2p⁶3s²3p⁶; Valence electrons: 8
Noble gases have a full outer shell of electrons, making them exceptionally unreactive.
Transition Metals (e.g., Group 6):
- Chromium (Cr): Electron configuration: [Ar]3d⁵4s¹; Valence electrons: 6 (Note the exception to the simple group rule)
- Molybdenum (Mo): Electron configuration: [Kr]4d⁵5s¹; Valence electrons: 6 (similar exception)
- Tungsten (W): Electron configuration: [Xe]4f¹⁴5d⁴6s²; Valence electrons: 6 (Again, an exception to the simple group rule)
The complexity in transition metals' valence electrons highlights the limitations of the simplified group number method. The multiple oxidation states of these elements demonstrate the variable participation of d electrons in chemical bonding.
Advanced Concepts and Exceptions
While the methods described above are generally reliable, several complexities and exceptions warrant further discussion:
-
Expanded Octet: Some elements in the third period and beyond can accommodate more than eight electrons in their valence shell due to the availability of vacant d orbitals. This is particularly common for elements like phosphorus and sulfur.
-
Incomplete Octet: Some elements, particularly boron and beryllium, may form stable compounds with fewer than eight valence electrons.
-
Odd Number of Valence Electrons: Elements with an odd number of valence electrons, such as nitrogen, often form covalent bonds to share electrons and achieve a more stable configuration.
Conclusion
Determining the number of valence electrons is a crucial skill in chemistry. While the group number on the periodic table offers a quick estimation for many main group elements, utilizing electron configurations provides a more precise and comprehensive method. Understanding the relationship between valence electrons and chemical bonding is fundamental to comprehending the reactivity and properties of elements and their compounds. Recognizing the exceptions and advanced concepts strengthens the foundation for deeper study in chemical bonding and reactivity. This knowledge underpins a wide range of chemical phenomena and applications. Remember that understanding valence electrons is a cornerstone of many chemical concepts, and mastering this foundational topic will significantly enhance your understanding of chemistry as a whole.
Latest Posts
Latest Posts
-
Ice Has A Lower Density Than Water Because Ice
Apr 09, 2025
-
Found In Both Prokaryotic And Eukaryotic Cells
Apr 09, 2025
-
Identify The Substrate In The Following Reaction
Apr 09, 2025
-
Which Of The Following Is Not A Characteristic Of Inflammation
Apr 09, 2025
-
Match The Regeneration Capacity Of The Following Tissues
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.