Number Of Hydrogen Bonds Between Guanine And Cytosine
News Leon
Mar 23, 2025 · 6 min read
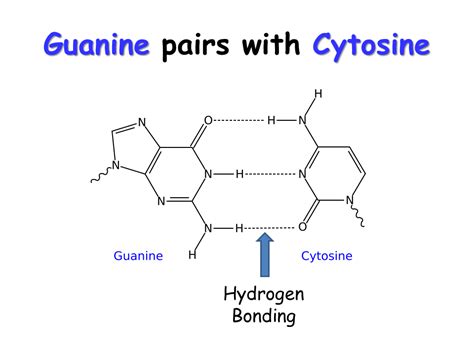
Table of Contents
The Intricate Dance of Guanine and Cytosine: Unveiling the Hydrogen Bonds
The elegance of DNA's double helix lies not only in its structure but also in the precise pairing of its constituent bases: adenine with thymine, and guanine with cytosine. This base pairing, governed by the formation of hydrogen bonds, is fundamental to DNA's stability, replication, and its role as the blueprint of life. While the adenine-thymine pair forms two hydrogen bonds, the guanine-cytosine (G-C) pair boasts a stronger connection, featuring three hydrogen bonds. This seemingly small difference in bond number has profound implications for DNA's properties and function. This article will delve deep into the specifics of these hydrogen bonds, exploring their formation, strength, and significance in the larger context of molecular biology.
Understanding Hydrogen Bonds: The Foundation of Base Pairing
Before we delve into the specifics of G-C base pairing, it's crucial to establish a clear understanding of hydrogen bonds themselves. These are a special type of dipole-dipole attraction between molecules, not a true covalent bond. They arise from the interaction between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom in a different molecule. This electronegativity difference creates a partial positive charge (δ+) on the hydrogen and a partial negative charge (δ-) on the electronegative atom. The electrostatic attraction between these opposite partial charges forms the hydrogen bond.
Hydrogen bonds are weaker than covalent bonds but are significantly stronger than other intermolecular forces like van der Waals forces. Their strength is crucial in biological systems, providing the necessary stability for structures like DNA's double helix while allowing for flexibility during processes like DNA replication and transcription.
The Three Hydrogen Bonds in Guanine-Cytosine Pairing: A Detailed Look
The interaction between guanine and cytosine is characterized by the formation of three hydrogen bonds. These bonds are strategically positioned between specific atoms on each base:
-
Bond 1: This bond occurs between the oxygen atom (O) of the carbonyl group in guanine and the nitrogen atom (N) of the amino group in cytosine. The oxygen atom carries a partial negative charge, and the nitrogen atom carries a partial positive charge, leading to a strong electrostatic attraction.
-
Bond 2: The second hydrogen bond forms between the nitrogen atom (N) in guanine's amino group and the nitrogen atom (N) in cytosine’s ring. Again, these atoms possess partial opposite charges, facilitating the hydrogen bond formation.
-
Bond 3: The third and final hydrogen bond is formed between the nitrogen atom (N) in guanine's ring and the nitrogen atom (N) of the amino group in cytosine. This bond further stabilizes the G-C base pair.
The precise arrangement and number of these hydrogen bonds ensure that guanine and cytosine are perfectly complementary, fitting together like pieces of a puzzle. The specificity of these interactions is vital for the accurate replication and transcription of genetic information.
The Significance of Three Hydrogen Bonds: Implications for DNA Stability and Function
The presence of three hydrogen bonds in the G-C pair compared to the two in the A-T pair has significant consequences for DNA's properties:
-
Increased Stability: The additional hydrogen bond in the G-C pair contributes to a stronger and more stable base pair compared to the A-T pair. This increased stability is crucial for maintaining the integrity of the DNA double helix, particularly under conditions of high temperature or extreme pH.
-
Melting Temperature: The higher number of hydrogen bonds in G-C rich DNA sequences results in a higher melting temperature (Tm). The Tm is the temperature at which 50% of the DNA strands are separated. DNA sequences with a higher G-C content require more energy (higher temperature) to denature, reflecting the increased stability of the G-C base pairs. This property is exploited in various molecular biology techniques, including polymerase chain reaction (PCR).
-
Regulation of Gene Expression: The stability of G-C base pairs can influence the accessibility of DNA to transcription factors and other regulatory proteins. Regions of DNA with a high G-C content may be less accessible, influencing the regulation of gene expression.
-
DNA Replication Fidelity: The precise and specific pairing between guanine and cytosine, facilitated by the three hydrogen bonds, ensures high fidelity during DNA replication. The accurate pairing of bases is critical for maintaining the integrity of the genetic code and preventing mutations.
Variations and Exceptions: Exploring the Nuances of G-C Base Pairing
While the canonical G-C base pair involves three hydrogen bonds, there are exceptions and variations that can occur under specific circumstances.
-
Tautomerization: Guanine and cytosine, like other bases, can exist in different tautomeric forms. These forms can alter the hydrogen bonding potential, leading to mispairing. While rare, tautomerization can contribute to spontaneous mutations.
-
Hoogsteen base pairing: Under specific conditions, guanine and cytosine can also form a Hoogsteen base pair, characterized by a different hydrogen bonding pattern compared to the standard Watson-Crick pairing. This type of pairing is observed in certain DNA structures and may play a role in specific biological processes.
-
Non-canonical base pairing: In some contexts, particularly in RNA structures, non-canonical base pairing can occur between guanine and cytosine, involving different hydrogen bond arrangements. These non-canonical interactions contribute to the complex three-dimensional structures formed by RNA molecules.
Beyond the Bonds: The Broader Context of G-C Base Pairing
The significance of the three hydrogen bonds in the G-C pair extends beyond the mere stability of the DNA double helix. These bonds play a crucial role in various biological processes:
-
DNA Replication: The precise pairing of guanine and cytosine during DNA replication is fundamental for the accurate copying of genetic information. DNA polymerase, the enzyme responsible for DNA replication, relies on the specific hydrogen bonding patterns to ensure the accurate incorporation of nucleotides during DNA synthesis.
-
DNA Transcription: Similarly, the precise pairing of guanine and cytosine is essential for accurate transcription, the process of synthesizing RNA molecules from DNA templates. RNA polymerase, the enzyme responsible for transcription, relies on the base pairing to ensure the accurate synthesis of RNA molecules.
-
DNA Repair: When DNA damage occurs, cellular repair mechanisms rely on the specific hydrogen bonding patterns to accurately identify and repair the damaged regions. The integrity of the G-C base pairs is crucial for maintaining the stability and function of the genome.
-
Genome stability and evolution: The higher stability of G-C base pairs contributes to the overall stability of the genome and can influence the rate of mutations and evolutionary changes.
Conclusion: The Unsung Heroes of Genetic Stability
The seemingly simple interaction between guanine and cytosine, mediated by three precise hydrogen bonds, is a cornerstone of life's molecular machinery. The increased stability provided by this stronger bond compared to the A-T pair has far-reaching implications for DNA's structural integrity, replication fidelity, and the regulation of gene expression. Understanding the intricacies of G-C base pairing is crucial for comprehending fundamental biological processes and advancing our knowledge of genetics, molecular biology, and medicine. The precise number of hydrogen bonds – three – is not merely a detail; it is a fundamental aspect of life's design, ensuring the fidelity and stability of the genetic information that defines us. Further research into the nuances of G-C base pairing will undoubtedly continue to reveal new insights into the complexity and elegance of life's molecular mechanisms.
Latest Posts
Latest Posts
-
The Maximum Population A Habitat Can Support Is Its
Mar 25, 2025
-
Half A Percent As A Decimal
Mar 25, 2025
-
The Figure Shows A Rectangular 20 Turn Coil Of Wire
Mar 25, 2025
-
A Group Of 8 Bits Is Called
Mar 25, 2025
-
85 Is 80 Of What Number
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Hydrogen Bonds Between Guanine And Cytosine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
