Number Of Electrons In Carbon Atom
News Leon
Mar 16, 2025 · 5 min read
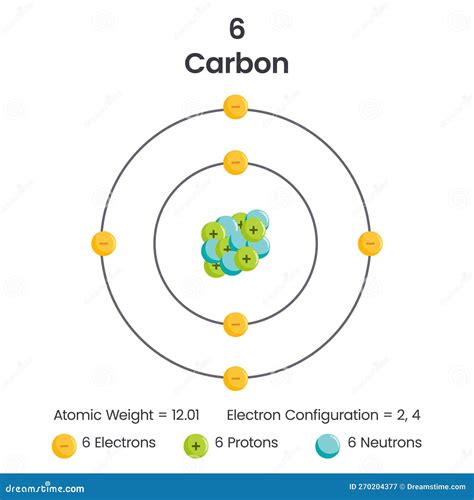
Table of Contents
Delving Deep into the Electron Count of a Carbon Atom
The seemingly simple question, "How many electrons does a carbon atom have?" opens a door to a fascinating exploration of atomic structure, quantum mechanics, and the very foundation of chemistry. While the answer itself is straightforward, understanding the why behind that number requires a dive into the intricacies of electron shells, orbitals, and the periodic table. This comprehensive guide will not only answer the question but also provide a robust understanding of the electron configuration of carbon and its implications.
The Straightforward Answer: Six Electrons
A neutral carbon atom possesses six electrons. This fundamental fact is directly derived from its atomic number, which is also six. The atomic number of an element represents the number of protons in its nucleus, and in a neutral atom, the number of protons equals the number of electrons, ensuring a balanced electrical charge.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
To truly grasp the significance of carbon's six electrons, we need to revisit the basic building blocks of an atom:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutral particles also located in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. Their arrangement determines an atom's chemical properties.
Carbon, with its six protons, inherently needs six electrons to maintain electrical neutrality. This balance is crucial for the atom's stability and its ability to interact with other atoms.
Electron Shells and Subshells: The Orbital Dance
Electrons don't randomly orbit the nucleus; they occupy specific energy levels called shells. These shells are further divided into subshells, which are composed of orbitals. Each orbital can hold a maximum of two electrons, following the Pauli Exclusion Principle.
The electron configuration of carbon reflects this organized arrangement:
- First shell (n=1): This shell contains only one subshell, the s subshell, which can hold a maximum of two electrons. In carbon, this shell is completely filled.
- Second shell (n=2): This shell contains two subshells: the s subshell (holding up to two electrons) and the p subshell (holding up to six electrons). In carbon, the s subshell is filled, and the p subshell has two electrons.
Therefore, the complete electron configuration of carbon is 1s²2s²2p². This notation concisely describes the occupancy of each subshell. The superscript indicates the number of electrons in each subshell.
Deeper Dive into Orbitals: Shapes and Electron Probability
Orbitals are not simply circular paths; they represent regions of space where there's a high probability of finding an electron. The s orbitals are spherical, while the p orbitals are dumbbell-shaped, oriented along the x, y, and z axes. Understanding orbital shapes is vital for comprehending molecular bonding and chemical reactivity.
The two electrons in the carbon's 2p subshell occupy two of the three available 2p orbitals, each electron occupying a separate orbital according to Hund's Rule of Maximum Multiplicity which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This arrangement maximizes the atom's overall stability.
The Significance of Carbon's Electron Configuration
Carbon's electron configuration is the cornerstone of its unique chemical properties and its central role in organic chemistry. The presence of four valence electrons (the electrons in the outermost shell) allows carbon to form four covalent bonds with other atoms. This ability to form stable bonds with itself and other elements is the basis for the incredible diversity of organic molecules, ranging from simple hydrocarbons to complex biomolecules like DNA and proteins.
Covalent Bonding: Sharing is Caring
Carbon's four valence electrons enable it to readily participate in covalent bonding, where atoms share electrons to achieve a stable electron configuration, typically resembling that of a noble gas (eight electrons in the outermost shell – the octet rule). This sharing of electrons creates strong bonds that hold atoms together in molecules. The tetrahedral geometry often exhibited by carbon-containing molecules directly results from this bonding pattern.
Hybrid Orbitals: A More Accurate Picture
While the simple electron configuration provides a good starting point, a more accurate depiction of carbon's bonding involves the concept of hybrid orbitals. In many carbon compounds, the 2s and 2p orbitals hybridize to form four equivalent sp³ hybrid orbitals, each participating in a covalent bond. This hybridization leads to a stable tetrahedral structure, commonly observed in molecules like methane (CH₄).
Isotopes of Carbon: Variations in Neutron Count
While the number of electrons in a neutral carbon atom is always six, the number of neutrons can vary, leading to different isotopes of carbon. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotopes are:
- Carbon-12 (¹²C): Six protons and six neutrons. This is the most abundant isotope.
- Carbon-13 (¹³C): Six protons and seven neutrons. This isotope is used in various scientific applications, including nuclear magnetic resonance (NMR) spectroscopy.
- Carbon-14 (¹⁴C): Six protons and eight neutrons. This radioactive isotope is used in radiocarbon dating to determine the age of organic materials.
It's crucial to remember that the number of electrons remains consistent across all carbon isotopes in their neutral state. The variations in neutron count affect only the atom's mass and stability, not its electronic structure and chemical properties.
Conclusion: The Importance of Six Electrons
The seemingly simple answer – six electrons – unveils a rich tapestry of atomic structure, quantum mechanics, and chemical bonding. Carbon's six electrons, with their specific arrangement in shells and orbitals, are the driving force behind its unparalleled ability to form a vast array of molecules, making it the backbone of life and a cornerstone of chemistry and material science. Understanding the electron count and configuration is foundational to comprehending the diversity and complexity of the carbon-based world around us. Further exploration into the nuances of atomic orbitals, molecular geometry, and bonding theories will only enhance this fundamental understanding.
Latest Posts
Latest Posts
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In Carbon Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
