Naoh Is Strong Or Weak Base
News Leon
Apr 01, 2025 · 6 min read
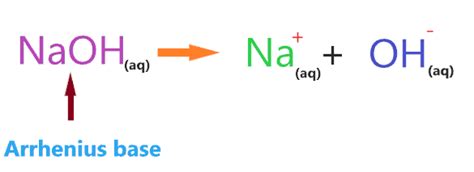
Table of Contents
NaOH: A Strong Base – Understanding its Properties and Reactions
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a strong base. This seemingly simple statement encapsulates a wealth of chemical properties and reactions that are crucial in various industrial processes and even everyday applications. Understanding the strength of a base, and NaOH's position within that classification, is fundamental to comprehending its behavior and safe handling. This article delves deep into the properties of NaOH, explaining why it's classified as a strong base, exploring its reactions, and highlighting its significant applications.
Defining Strong and Weak Bases
Before diving into the specifics of NaOH, let's establish a clear understanding of the difference between strong and weak bases. This distinction lies in their degree of dissociation in aqueous solutions.
-
Strong bases: These bases completely dissociate into their constituent ions (cations and hydroxide anions, OH⁻) when dissolved in water. This means that virtually every molecule of the base reacts with water to produce hydroxide ions. The resulting solution has a high concentration of OH⁻ ions, leading to a high pH value.
-
Weak bases: These bases only partially dissociate in water. Only a small fraction of the base molecules react with water to form hydroxide ions. The equilibrium lies significantly towards the undissociated base, resulting in a lower concentration of OH⁻ ions and a lower pH compared to a strong base of the same concentration.
Why NaOH is a Strong Base: Complete Dissociation
The defining characteristic of NaOH as a strong base is its complete dissociation in water. The reaction can be represented as follows:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
When solid NaOH is added to water, it dissolves completely, breaking apart into sodium cations (Na⁺) and hydroxide anions (OH⁻). There are essentially no undissociated NaOH molecules left in the solution. This complete dissociation is the reason behind its strong basicity and its ability to readily increase the pH of a solution.
Understanding the Dissociation Process
The complete dissociation of NaOH stems from the strength of the O-H bond in the hydroxide ion (OH⁻). The sodium-oxygen bond (Na-O) is relatively weak and easily broken in the presence of water molecules. The highly electronegative oxygen atom in the OH⁻ ion strongly attracts the hydrogen atoms from water molecules, further stabilizing the ions in solution. This interaction leads to the complete separation of the Na⁺ and OH⁻ ions, leading to its complete ionization.
Properties of NaOH: More Than Just a Strong Base
NaOH's strength as a base is only one aspect of its multifaceted properties. Other important characteristics include:
-
Highly corrosive: NaOH is extremely corrosive to many materials, including skin, eyes, and mucous membranes. It can cause severe burns and tissue damage upon contact. Appropriate safety precautions, including the use of protective gear, are crucial when handling NaOH.
-
Hygroscopic: NaOH readily absorbs moisture from the air, a property known as hygroscopy. This can lead to the formation of a concentrated solution, further increasing its corrosive nature. Proper storage in airtight containers is essential to prevent this.
-
High solubility: NaOH is highly soluble in water, readily dissolving to form alkaline solutions. The solubility increases with temperature.
-
White crystalline solid: In its pure form, NaOH is a white crystalline solid. However, commercially available NaOH often appears as pellets or flakes due to the manufacturing process.
Reactions of NaOH: A Versatile Reactant
The strong basicity of NaOH makes it a highly reactive substance, participating in various chemical reactions. Some key reactions include:
1. Neutralization Reactions:
NaOH readily reacts with acids to form salts and water. This is a classic acid-base neutralization reaction. For instance, the reaction with hydrochloric acid (HCl) is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This reaction is exothermic, meaning it releases heat. The heat generated can be significant, especially with concentrated solutions.
2. Reactions with Metal Oxides and Hydroxides:
NaOH can react with certain metal oxides and hydroxides to form corresponding salts. For example, the reaction with aluminum oxide (Al₂O₃) is:
2NaOH(aq) + Al₂O₃(s) → 2NaAlO₂(aq) + H₂O(l)
This reaction is often utilized in the aluminum industry.
3. Esterification and Saponification:
NaOH plays a crucial role in both esterification and saponification reactions. In saponification, it reacts with fats and oils to produce soap and glycerol. This reaction is historically significant and forms the basis of traditional soap making.
4. Reactions with Organic Compounds:
NaOH reacts with various organic compounds, influencing their chemical properties and structure. For instance, it can be used to deprotonate acidic organic compounds or catalyze certain organic reactions.
Applications of NaOH: A Wide Range of Uses
The diverse properties and reactions of NaOH make it an essential chemical in a wide range of industries and applications:
-
Chemical Industry: NaOH is a fundamental building block in numerous chemical manufacturing processes, including the production of soaps, detergents, paper, textiles, and dyes.
-
Pulp and Paper Industry: NaOH is extensively used in the Kraft process for the pulping of wood, separating cellulose fibers from lignin.
-
Water Treatment: NaOH is employed to adjust the pH of water, improving its quality and removing impurities.
-
Food Industry: Although indirectly, NaOH is used in food processing to adjust pH levels or act as a food additive (with regulations).
-
Metal Industries: NaOH is utilized for various purposes such as cleaning metals, etching, and in aluminum production.
-
Drain Cleaners: Many commercial drain cleaners contain NaOH, due to its ability to dissolve fats, oils, and other organic substances causing blockages. Caution: Use of drain cleaners requires adherence to safety precautions due to its corrosive nature.
Safety Precautions When Handling NaOH
Due to its corrosive nature, handling NaOH requires stringent safety measures:
-
Protective gear: Always wear appropriate protective gear, including gloves, eye protection, and lab coats.
-
Ventilation: Ensure adequate ventilation when handling NaOH to prevent inhalation of dust or fumes.
-
Emergency response: Have emergency eyewash stations and safety showers readily available.
-
Storage: Store NaOH in airtight containers in a cool, dry place, away from incompatible materials.
-
Disposal: Dispose of NaOH according to local regulations and guidelines.
Conclusion: NaOH – A Powerful and Versatile Strong Base
Sodium hydroxide's strength as a base is a crucial factor determining its reactivity and applications. Its complete dissociation in water, leading to a high concentration of hydroxide ions, is the cornerstone of its diverse uses in various industries. However, its corrosive nature necessitates careful handling and adherence to strict safety protocols. Understanding the properties and reactions of NaOH is crucial for its safe and effective utilization in various fields, from industrial processes to everyday applications. Its versatility and chemical reactivity make it a pivotal chemical in the modern world, underlining its importance across multiple sectors. Continued research and development in safer handling and applications ensure its continued vital role in various industrial processes while prioritizing safety.
Latest Posts
Latest Posts
-
What Percent Of The Figure Is Shaded
Apr 02, 2025
-
Why Nh3 Is More Polar Than Nf3
Apr 02, 2025
-
Abraham Maslows Needs Theory Of Motivation Assumes That
Apr 02, 2025
-
Pb Oh 2 Hcl H2o Pbcl2
Apr 02, 2025
-
Is Ethanol More Polar Than Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Naoh Is Strong Or Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
