Nanh2 Is The Conjugate Base Of
News Leon
Mar 16, 2025 · 6 min read
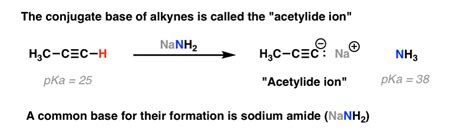
Table of Contents
NH₂⁻ is the Conjugate Base of: Understanding Brønsted-Lowry Acids and Bases
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves into the concept, focusing specifically on the amide ion, NH₂⁻, and identifying its conjugate acid. We'll explore the Brønsted-Lowry theory, delve into the properties of NH₂⁻, and examine its role in various chemical reactions. We'll also touch upon related concepts like pKa values and their significance in determining acid strength.
The Brønsted-Lowry Definition of Acids and Bases
Before identifying the conjugate acid of NH₂⁻, let's refresh our understanding of the Brønsted-Lowry theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. Unlike the Arrhenius theory, which limits acids and bases to aqueous solutions, the Brønsted-Lowry theory encompasses a broader range of reactions, including those in non-aqueous solvents.
A crucial aspect of the Brønsted-Lowry theory is the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton.
Identifying the Conjugate Acid of NH₂⁻
The amide ion, NH₂⁻, is a strong base. It readily accepts a proton. To determine its conjugate acid, we simply add a proton (H⁺) to the structure:
NH₂⁻ + H⁺ → NH₃
Therefore, the conjugate acid of NH₂⁻ is ammonia (NH₃). This is a straightforward application of the Brønsted-Lowry definition. The reaction shows that NH₂⁻ acts as a base by accepting a proton from an acid, thereby forming its conjugate acid, NH₃.
Properties of NH₂⁻ and its Conjugate Acid, NH₃
Understanding the properties of NH₂⁻ and NH₃ is crucial to understanding their roles in chemical reactions.
Properties of NH₂⁻ (Amide Ion)
- Strong Base: NH₂⁻ is a very strong base due to its high affinity for protons. The lone pair of electrons on the nitrogen atom readily accepts a proton.
- Highly Reactive: Its strong basicity makes it highly reactive, readily reacting with many acids, including water. Reactions with water are highly exothermic.
- Exists in Non-Aqueous Solvents: Due to its high reactivity with water, NH₂⁻ is typically found in non-aqueous solvents like liquid ammonia.
- Nucleophile: The lone pair on the nitrogen also makes NH₂⁻ a good nucleophile, participating in nucleophilic substitution and addition reactions.
Properties of NH₃ (Ammonia)
- Weak Base: While NH₃ is a base, it is significantly weaker than its conjugate base, NH₂⁻. It accepts protons but less readily.
- Weakly Alkaline Solution in Water: Ammonia forms a weakly alkaline solution in water due to the equilibrium reaction: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻.
- Gas at Room Temperature: Ammonia is a gas at standard temperature and pressure, characterized by a pungent odor.
- Versatile Reagent: Ammonia is a widely used reagent in both organic and inorganic chemistry, used in various synthesis reactions.
pKa Values and Acid Strength
The pKa value is a crucial measure of the strength of an acid. It represents the negative logarithm of the acid dissociation constant (Ka). A lower pKa value indicates a stronger acid. The relationship between an acid and its conjugate base is inversely proportional regarding their strengths: a strong acid has a weak conjugate base, and a weak acid has a strong conjugate base.
While the pKa of NH₂⁻ itself is not easily defined in aqueous solution due to its rapid reaction with water, we can consider the pKa of its conjugate acid, NH₃. The pKa of the ammonium ion (NH₄⁺, the conjugate acid of NH₃) is approximately 9.25. This indicates that NH₄⁺ is a relatively weak acid, consistent with NH₃ being a weak base, and NH₂⁻ being a strong base.
The significant difference in pKa values between NH₄⁺ and its conjugate base highlights the strength of the amide ion as a base.
Reactions Involving NH₂⁻ and NH₃
NH₂⁻ and NH₃ participate in numerous chemical reactions, showcasing their contrasting properties.
Reactions involving NH₂⁻
- Reaction with Water: NH₂⁻ reacts violently with water, acting as a strong base: NH₂⁻ + H₂O → NH₃ + OH⁻
- Reactions with other acids: NH₂⁻ readily reacts with other Brønsted acids, abstracting a proton.
- Nucleophilic Substitution and Addition: The nucleophilic nature of the amide ion allows it to participate in many organic reactions.
Reactions involving NH₃
- Formation of Ammonium Salts: Ammonia reacts with acids to form ammonium salts (e.g., NH₄Cl).
- Reaction with Water (Weak Base Behavior): As mentioned earlier, ammonia reacts weakly with water to form ammonium ions and hydroxide ions.
- Complex Formation: Ammonia can act as a ligand in the formation of coordination complexes with metal ions.
Applications of NH₂⁻ and NH₃
Both NH₂⁻ and NH₃ have significant applications in various fields.
Applications of NH₂⁻
The highly reactive nature of NH₂⁻ limits its direct applications. It's more commonly encountered as an intermediate in various chemical processes. Research into its use in specific organic synthesis is ongoing due to its strong nucleophilic properties.
Applications of NH₃
Ammonia has numerous applications:
- Fertilizer Production: Ammonia is a key ingredient in the production of nitrogen-based fertilizers, vital for agriculture.
- Industrial Applications: It's used in the production of nitric acid, nylon, and other chemicals.
- Refrigerant: In the past, ammonia was used as a refrigerant, although its use has decreased due to safety concerns.
- Cleaning Agent: Ammonia is a common household cleaning agent.
- Laboratory Reagent: It's a versatile reagent in various chemical synthesis reactions.
Safety Considerations
Both NH₂⁻ and NH₃ require careful handling due to their potential hazards.
Safety Considerations for NH₂⁻
NH₂⁻’s extreme reactivity with water necessitates handling in anhydrous conditions under an inert atmosphere to prevent dangerous exothermic reactions. Appropriate safety equipment, including gloves, eye protection, and a well-ventilated environment, are mandatory.
Safety Considerations for NH₃
While less reactive than NH₂⁻, ammonia is still hazardous. It’s a pungent gas that can irritate the eyes, nose, and throat. Proper ventilation is essential when handling ammonia. High concentrations can be toxic and even lethal.
Conclusion
The amide ion, NH₂⁻, is the conjugate base of ammonia (NH₃). Understanding this conjugate acid-base pair requires a firm grasp of the Brønsted-Lowry theory. The significant difference in their basicities, reflected in their pKa values, highlights the importance of considering the context and the reaction environment when discussing acid-base chemistry. Both NH₂⁻ and NH₃ have various applications, ranging from industrial production to laboratory research, but safety precautions must always be observed when handling these chemicals due to their inherent properties. Further research continues to explore the potential uses and reactions of both species, expanding our understanding of their crucial role in chemistry.
Latest Posts
Latest Posts
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Nanh2 Is The Conjugate Base Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
