Methods Of Purification In Organic Chemistry
News Leon
Mar 24, 2025 · 7 min read
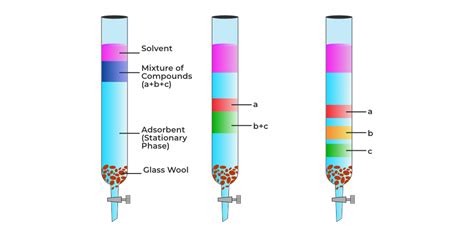
Table of Contents
Methods of Purification in Organic Chemistry
Organic chemistry, the study of carbon-containing compounds, often involves the synthesis of new molecules and the isolation of desired products from complex reaction mixtures. A crucial aspect of this field is the purification of organic compounds, ensuring the purity and characterization of the synthesized materials. Impurities can significantly affect the properties and reactivity of organic compounds, rendering them unusable for further research or application. Therefore, mastering various purification techniques is essential for any organic chemist. This comprehensive guide explores several common and effective methods used to purify organic compounds.
Why Purification is Crucial in Organic Chemistry
Before delving into the specific methods, it's crucial to understand why purification is paramount in organic chemistry. Impurities can lead to:
- Inaccurate experimental results: The presence of impurities can alter the physical properties (like melting point, boiling point) and chemical reactivity of a compound, leading to flawed experimental data and unreliable conclusions.
- Unreliable chemical reactions: Impurities can interfere with subsequent reactions, leading to unexpected or undesired products, reduced yields, and compromised reaction efficiency.
- Hazardous compounds: Some impurities can be toxic or even explosive, presenting significant safety risks during handling and further experimentation.
- Compromised product quality: In industrial applications, impurities can compromise the quality and performance of the final product, leading to economic losses and potential safety issues.
Therefore, rigorous purification is not merely a procedural step but a cornerstone of reliable research and safe practice in organic chemistry.
Common Methods of Purification
Several methods are employed to purify organic compounds, each suited to different types of compounds and levels of impurity. The choice of method depends on the physical properties of the compound, the nature of the impurities, and the desired level of purity.
1. Recrystallization
Recrystallization is a powerful technique that exploits the difference in solubility of a compound at different temperatures. It's particularly effective for purifying solid compounds. The process involves:
- Dissolving the impure compound: The solid is dissolved in a minimum amount of hot solvent. The choice of solvent is crucial; it should dissolve the compound well when hot but poorly when cold. The ideal solvent will dissolve impurities either very well or very poorly at all temperatures.
- Hot filtration: This step removes any insoluble impurities that may be present.
- Cooling and crystallization: The solution is slowly cooled, allowing the purified compound to crystallize out as the solubility decreases. Slow cooling promotes the formation of larger, purer crystals.
- Isolation and drying: The crystals are collected by filtration, washed with a small amount of cold solvent to remove any remaining impurities, and then dried.
Advantages: Relatively simple, effective for removing small amounts of impurities.
Disadvantages: Not suitable for compounds that decompose on heating or are very soluble in all solvents. Yield can be reduced if significant amounts of product are lost in the solution.
2. Distillation
Distillation separates compounds based on their boiling points. It's widely used for purifying liquids. Several types of distillation exist:
- Simple distillation: Suitable for separating liquids with significantly different boiling points (at least 70°C difference).
- Fractional distillation: Used to separate liquids with boiling points closer together. A fractionating column is used to increase the efficiency of separation.
- Vacuum distillation: Used for compounds with high boiling points which may decompose at atmospheric pressure. Reducing the pressure lowers the boiling point.
- Steam distillation: Used for volatile compounds that are immiscible with water. Steam is passed through the mixture, carrying the volatile compound over.
Advantages: Effective for separating liquids based on boiling points, relatively simple for simple distillations.
Disadvantages: Less effective for separating liquids with similar boiling points. Requires specialized equipment for fractional or vacuum distillation.
3. Extraction
Extraction utilizes the difference in solubility of a compound in two immiscible solvents. It's often used to separate organic compounds from aqueous solutions. The process generally involves:
- Adding the second solvent: The aqueous solution containing the organic compound is shaken with an immiscible organic solvent (e.g., diethyl ether, dichloromethane).
- Separation of layers: The two solvents form distinct layers based on their densities. The organic layer containing the dissolved organic compound is separated from the aqueous layer.
- Drying the organic layer: Any remaining water is removed from the organic layer using a drying agent like anhydrous sodium sulfate.
- Evaporation of the solvent: The solvent is evaporated to obtain the purified organic compound.
Advantages: Effective for separating organic compounds from aqueous solutions, can be used to remove impurities selectively.
Disadvantages: Requires careful handling of solvents, potential for loss of product during the transfer.
4. Chromatography
Chromatography is a powerful technique used to separate mixtures based on the differential distribution of the components between a stationary phase and a mobile phase. Several types of chromatography exist:
- Thin-layer chromatography (TLC): A simple and rapid technique used for qualitative analysis and monitoring reactions. It involves spotting the mixture on a thin layer of adsorbent (e.g., silica gel) and developing the chromatogram using a suitable solvent.
- Column chromatography: Used for preparative separation of larger quantities of compounds. The mixture is loaded onto a column packed with an adsorbent, and the components are separated by elution with a suitable solvent.
- High-performance liquid chromatography (HPLC): A sophisticated technique used for highly efficient separation of complex mixtures. It employs high pressure to force the mobile phase through a column packed with a stationary phase.
- Gas chromatography (GC): Used to separate volatile compounds. The mixture is vaporized and carried through a column by an inert gas.
Advantages: Highly versatile, can separate complex mixtures with high efficiency.
Disadvantages: Can be time-consuming and requires specialized equipment, especially for HPLC and GC.
5. Sublimation
Sublimation is a purification technique that involves the transition of a solid directly to the gaseous phase without passing through the liquid phase. It's particularly useful for purifying solids that sublime easily, while impurities remain in the solid phase. The process generally involves heating the impure solid in a sublimation apparatus, causing it to sublime, and then cooling the vapor to deposit the purified solid.
Advantages: Effective for purifying solids that sublime easily, leaving impurities behind.
Disadvantages: Limited to compounds that sublime readily and have volatile impurities that do not sublime.
Choosing the Appropriate Purification Method
The selection of the most appropriate purification method depends on several factors:
- The nature of the compound: Is it a solid or a liquid? What is its melting point and boiling point? Is it volatile? Is it sensitive to heat or air?
- The nature of the impurities: What are the types and amounts of impurities present? Are the impurities soluble or insoluble in the same solvents?
- The desired level of purity: How pure does the compound need to be for its intended application?
- The scale of the purification: How much material needs to be purified?
Often, a combination of methods is necessary to achieve the desired level of purity. For instance, extraction might be followed by recrystallization, or distillation might be combined with chromatography.
Modern Advancements in Purification Techniques
Recent advancements in analytical chemistry and separation science have led to the development of more sophisticated purification techniques:
- Supercritical fluid chromatography (SFC): This method utilizes supercritical fluids as the mobile phase, offering advantages in terms of speed, efficiency, and environmental friendliness.
- Preparative HPLC: With the advances in column technology and detectors, preparative HPLC now allows for efficient purification of larger quantities of compounds.
- Flash chromatography: This automated form of column chromatography uses pressurized gas to speed up the process and improve efficiency.
- Membrane separation techniques: These techniques utilize membranes to separate compounds based on size or other properties, offering advantages in terms of scalability and automation.
Conclusion
Purification techniques are indispensable tools in the organic chemist's arsenal. Mastering these methods is crucial for obtaining pure compounds, ensuring accurate experimental results, and advancing the field of organic chemistry. The choice of method depends on the specific circumstances, but understanding the principles behind each technique is key to effectively purifying organic compounds. As technology advances, new and improved purification methods will continue to emerge, pushing the boundaries of what is possible in organic synthesis and analysis. The constant pursuit of purity remains a vital aspect of successful organic chemistry research and industrial applications.
Latest Posts
Related Post
Thank you for visiting our website which covers about Methods Of Purification In Organic Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.