Isotopes Have A Different Number Of
News Leon
Mar 16, 2025 · 6 min read
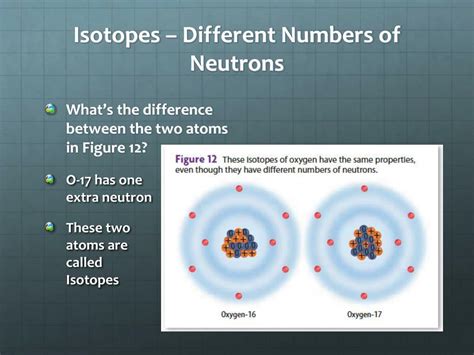
Table of Contents
Isotopes: A Deep Dive into Atoms with Different Neutron Counts
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly small difference has profound implications for the element's properties, applications, and even the history of the universe. Understanding isotopes is crucial for comprehending a wide range of scientific fields, from nuclear physics and chemistry to geology, medicine, and environmental science. This comprehensive article explores the intricacies of isotopes, delving into their properties, applications, and the significance of their varying neutron counts.
The Atomic Nucleus: Protons, Neutrons, and Isotopes
At the heart of every atom lies the nucleus, a densely packed region containing protons and neutrons. Protons carry a positive electrical charge, while neutrons are electrically neutral. The number of protons defines the atomic number of an element and determines its chemical identity. For example, all atoms with one proton are hydrogen, regardless of any other variations.
However, the number of neutrons in an atom's nucleus can vary. This variation, while not altering the element's chemical identity (its proton number), creates different isotopes of that element. These isotopes are identified by their mass number, which is the sum of the protons and neutrons in the nucleus.
For example, consider carbon:
- Carbon-12 (¹²C): Contains 6 protons and 6 neutrons (mass number = 12). This is the most abundant isotope of carbon.
- Carbon-13 (¹³C): Contains 6 protons and 7 neutrons (mass number = 13). A stable isotope used in various scientific applications.
- Carbon-14 (¹⁴C): Contains 6 protons and 8 neutrons (mass number = 14). A radioactive isotope with a half-life of approximately 5,730 years, crucial in radiocarbon dating.
The differing neutron counts are what distinguish these isotopes, leading to subtle yet significant differences in their physical and nuclear properties.
Isotope Properties: Mass, Stability, and Radioactivity
The primary difference between isotopes stems from their varying neutron numbers, influencing their:
1. Mass:
The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus, as electrons contribute negligible mass. Isotopes with more neutrons have a greater mass than those with fewer neutrons. This mass difference is crucial in many analytical techniques, such as mass spectrometry, used to separate and identify isotopes.
2. Nuclear Stability:
The neutron-to-proton ratio plays a critical role in determining the stability of an atomic nucleus. For lighter elements, a 1:1 ratio is generally stable. However, as the atomic number increases, a higher neutron-to-proton ratio is needed for stability. Isotopes with unstable nuclei are radioactive, meaning they undergo spontaneous nuclear decay to achieve a more stable configuration.
3. Radioactivity:
Radioactive isotopes emit radiation in the form of alpha particles, beta particles, or gamma rays during decay. This radioactive decay can transform the isotope into a different element or a more stable isotope of the same element. The rate of decay is characterized by the isotope's half-life, which is the time it takes for half of the initial amount of the isotope to decay.
The radioactivity of certain isotopes makes them invaluable tools in various applications, including medical imaging (e.g., PET scans using ¹⁸F), cancer therapy (e.g., cobalt-60), and geological dating (e.g., uranium-238 in uranium-lead dating).
Applications of Isotopes Across Diverse Fields
The unique properties of isotopes make them indispensable tools in a wide array of fields:
1. Medicine:
- Radioactive tracers: Radioactive isotopes are used as tracers to follow the movement of substances within the body. This technique is crucial for medical imaging, diagnosing diseases, and monitoring treatment effectiveness.
- Radiation therapy: Radioactive isotopes like cobalt-60 and iodine-131 are employed in radiation therapy to target and destroy cancerous cells.
- Nuclear medicine: Various radioactive isotopes are used in nuclear medicine for diagnostic and therapeutic purposes, including PET scans, SPECT scans, and radiotherapy.
2. Industry:
- Radioactive dating: Isotopes such as carbon-14 and uranium-238 are used to determine the age of artifacts, fossils, and geological formations.
- Industrial tracers: Isotopes are used to trace the flow of materials in industrial processes, optimizing efficiency and identifying leaks or blockages.
- Sterilization: Gamma radiation from radioactive isotopes is used to sterilize medical equipment, food, and other products.
3. Environmental Science:
- Environmental monitoring: Isotopes are used to track pollution sources, monitor water movement, and study atmospheric processes.
- Climate change research: Isotopic analysis is crucial for studying past and present climate changes and predicting future trends.
- Ecological studies: Isotopes provide valuable insights into the food chains, migration patterns, and ecological interactions of organisms.
4. Geology and Archaeology:
- Geochronology: Radioactive isotopes like uranium-238 and potassium-40 are crucial for determining the age of rocks and minerals.
- Paleoclimatology: Isotope analysis of ice cores and sediments provides insights into past climates and environmental changes.
- Archaeological dating: Carbon-14 dating is a cornerstone of archaeology, allowing researchers to determine the age of organic materials.
Isotope Separation: Techniques and Applications
Separating isotopes is often challenging due to their near-identical chemical properties. However, the slight mass difference between isotopes can be exploited using various separation techniques:
- Mass spectrometry: This technique separates isotopes based on their mass-to-charge ratio, providing precise measurements of isotopic abundance.
- Gas diffusion: This method utilizes the slightly different diffusion rates of gases containing different isotopes.
- Centrifugation: This technique uses centrifugal force to separate isotopes based on their mass differences.
- Laser isotope separation: This advanced technique uses lasers to selectively excite and ionize specific isotopes, enabling their separation.
Isotope separation has numerous applications, including:
- Nuclear fuel enrichment: Uranium enrichment, a crucial step in nuclear power generation, relies on separating uranium-235 from uranium-238.
- Medical isotope production: Separation techniques are used to isolate specific radioactive isotopes needed for medical applications.
- Scientific research: Separated isotopes are essential for various scientific experiments and analyses.
The Significance of Isotopes: A Broader Perspective
The study of isotopes has far-reaching implications, extending beyond the specific applications mentioned above. Understanding isotopic ratios provides crucial insights into:
- The origin and evolution of the universe: Isotopic abundances in meteorites and stars provide clues about the processes that shaped the early universe.
- The formation and evolution of planets: Isotopic ratios in planetary materials reveal information about the formation and geological history of planets.
- Biological processes: Isotope analysis reveals insights into metabolic pathways, nutrient cycling, and ecological interactions in biological systems.
The continuing research and development in isotope science promise further advancements and applications in various fields, paving the way for a deeper understanding of the universe and our place within it. The subtle differences in neutron counts within atomic nuclei hold immense significance, impacting our understanding of fundamental scientific principles and providing invaluable tools for solving real-world problems. From medical diagnostics to archaeological dating, the applications of isotopes continue to evolve, making it a vibrant and dynamic field of scientific inquiry. The ability to separate and analyze isotopes is a testament to human ingenuity and its capacity to harness the power of nature for the advancement of knowledge and technology. As we delve deeper into the intricacies of the atomic nucleus, the potential applications of isotopes are sure to continue expanding, enriching our understanding of the world around us.
Latest Posts
Latest Posts
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Have A Different Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
