Is Water Freezing Endothermic Or Exothermic
News Leon
Mar 24, 2025 · 5 min read
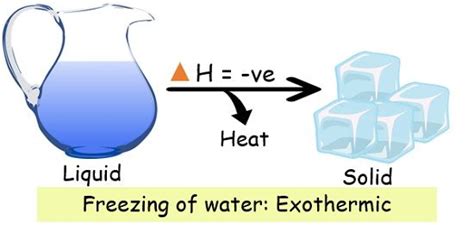
Table of Contents
Is Water Freezing Endothermic or Exothermic? Understanding Enthalpy Changes in Phase Transitions
The question of whether water freezing is endothermic or exothermic often trips up students new to thermodynamics. The seemingly simple process of water turning to ice hides a subtle yet crucial concept: the difference between heat transfer and enthalpy change. This article delves deep into the thermodynamics of water freezing, clarifying the misconception and providing a comprehensive understanding of the underlying principles.
Understanding Endothermic and Exothermic Processes
Before diving into the specifics of water freezing, let's establish a firm grasp on the definitions of endothermic and exothermic processes. These terms describe the direction of heat flow relative to the system undergoing a change.
-
Exothermic Processes: These processes release heat to the surroundings. The system's internal energy decreases, and the surroundings become warmer. Think of burning wood – it releases heat into the environment. The enthalpy change (ΔH) for an exothermic reaction is negative.
-
Endothermic Processes: These processes absorb heat from the surroundings. The system's internal energy increases, and the surroundings become cooler. Melting ice is a classic example; it absorbs heat from its surroundings to change phase. The enthalpy change (ΔH) for an endothermic reaction is positive.
The Enthalpy of Fusion: A Key Concept
The process of water freezing involves a phase transition – a change in the physical state of matter. Specifically, it's a transition from the liquid phase (water) to the solid phase (ice). The enthalpy change associated with this phase transition is called the enthalpy of fusion (ΔH<sub>fus</sub>), also known as the latent heat of fusion. This represents the amount of heat absorbed or released when a substance melts or freezes.
For water, the enthalpy of fusion is positive when melting (ice to water) and negative when freezing (water to ice). This seemingly contradictory point is the crux of the often-misunderstood nature of this process.
Water Freezing: An Exothermic Process
Water freezing is an exothermic process. This means that when liquid water transitions to solid ice, it releases heat into its surroundings. The molecules in liquid water possess higher kinetic energy than those in ice. As water freezes, these molecules lose kinetic energy, and this energy is released as heat. This is why the temperature of the surroundings slightly increases during the freezing process. The enthalpy change (ΔH<sub>fus</sub>) for water freezing is negative.
The Molecular Perspective
To understand this at a molecular level, consider the arrangement of water molecules in liquid and solid states:
-
Liquid Water: Water molecules are relatively mobile and move freely, interacting with each other through hydrogen bonds that are constantly breaking and reforming.
-
Ice: Water molecules arrange themselves into a highly ordered crystalline structure, where hydrogen bonds are more stable and fixed. This ordered structure requires less kinetic energy; the energy difference is released as heat.
The formation of these stronger, more stable hydrogen bonds in the ice lattice is the key to understanding the exothermic nature of water freezing. This energy release is manifested as heat transferred to the surroundings.
The Role of Temperature and Heat Transfer
It's crucial to distinguish between the temperature of the water and the heat transfer involved in the freezing process. While the temperature of the water remains constant at 0°C during the phase transition, heat is still being released to the surroundings. This heat is the latent heat of fusion. The temperature doesn't drop further until all the water has solidified. This constant temperature during phase change is a characteristic of the latent heat.
The system (the water) is losing energy (heat) to its surroundings, hence it's exothermic. It's tempting to assume that because the temperature remains constant, there's no heat transfer. This is incorrect. The heat transfer is happening; it's just that it's not reflected in a change of temperature. Instead, it's used to rearrange the water molecules into the crystalline structure of ice.
Common Misconceptions and Clarifications
The confusion often stems from the following:
-
Focusing solely on the temperature: Observing that the temperature doesn't decrease during freezing can lead to the incorrect conclusion that the process is endothermic. Remember, the energy is released as latent heat.
-
Confusing heat transfer with enthalpy change: While heat is released to the surroundings (heat transfer), the crucial factor is the enthalpy change of the system. For freezing water, the enthalpy change (ΔH<sub>fus</sub>) is negative.
-
Ignoring the system's perspective: The question needs to be approached from the perspective of the system undergoing the change (the water). The water is losing energy to the surroundings, making the process exothermic.
Real-World Applications of Understanding Water Freezing
Understanding the exothermic nature of water freezing has far-reaching applications:
-
Ice Formation in Weather: The release of heat during freezing plays a crucial role in weather patterns. The latent heat released during freezing moderates temperature changes in cold climates.
-
Food Preservation: Freezing food relies on the exothermic heat release to lower the food's temperature and inhibit microbial growth.
-
Industrial Processes: Many industrial processes utilize controlled freezing to achieve desired outcomes, relying on the heat released during the process.
-
Cryopreservation: The controlled freezing of biological materials for long-term storage depends on managing the heat released during the freezing process.
Conclusion: Freezing Water – A Negative Enthalpy Change
In conclusion, water freezing is unequivocally an exothermic process. Despite the constant temperature during the phase transition, the system (the water) releases heat to its surroundings, resulting in a negative enthalpy change (ΔH<sub>fus</sub>). This subtle but crucial distinction between heat transfer and enthalpy change is key to understanding the thermodynamics of phase transitions. The release of this latent heat plays a significant role in various natural phenomena and industrial processes, highlighting the importance of a clear understanding of this fundamental thermodynamic concept. Understanding this principle extends our knowledge across diverse fields, from meteorology to biology and engineering. By understanding the process from the system's perspective and recognizing the role of latent heat, we can accurately categorize water freezing as the exothermic process it truly is.
Latest Posts
Related Post
Thank you for visiting our website which covers about Is Water Freezing Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.