Is Uracil A Pyrimidine Or Purine
News Leon
Mar 23, 2025 · 6 min read
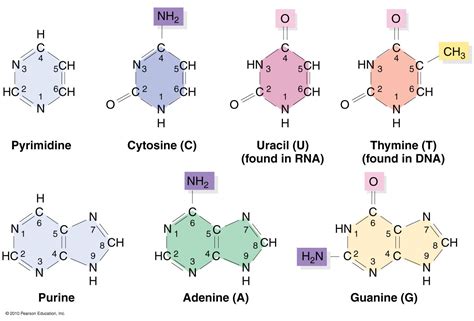
Table of Contents
Is Uracil a Pyrimidine or Purine? A Deep Dive into Nucleic Acid Bases
Understanding the fundamental building blocks of life is crucial for comprehending biological processes. Nucleic acids, DNA and RNA, are the blueprints of life, and their structure hinges on the precise arrangement of nitrogenous bases. One such base, uracil, often sparks questions regarding its classification. This comprehensive article will definitively answer the question: Is uracil a pyrimidine or a purine? We'll delve into the chemical structure, biological roles, and comparisons with other bases to solidify your understanding.
Understanding the Basics: Pyrimidines and Purines
Before classifying uracil, let's establish a clear understanding of pyrimidines and purines. These are two distinct classes of nitrogenous bases that form the core of nucleic acids. The difference lies in their ring structures:
Pyrimidines: The Single Ring Structure
Pyrimidines are characterized by a single six-membered heterocyclic aromatic ring containing two nitrogen atoms. Think of it as a single ring with two nitrogens embedded within the carbon ring. Key pyrimidines found in nucleic acids include:
- Cytosine (C): Found in both DNA and RNA.
- Thymine (T): Found exclusively in DNA.
- Uracil (U): Found exclusively in RNA.
Purines: The Double Ring Structure
Purines, on the other hand, possess a more complex structure comprising a six-membered ring fused to a five-membered ring. This double-ring system contains four nitrogen atoms. The purine bases vital to nucleic acids are:
- Adenine (A): Found in both DNA and RNA.
- Guanine (G): Found in both DNA and RNA.
Uracil: A Definitive Classification
Now, armed with the knowledge of pyrimidine and purine structures, we can definitively answer the central question: Uracil is a pyrimidine. Its chemical structure perfectly aligns with the pyrimidine definition: a single six-membered ring with two nitrogen atoms. There's no fusion of rings like in purines. This structural characteristic dictates its role and interactions within RNA molecules.
The Chemical Structure of Uracil: A Closer Look
The chemical formula of uracil is C₄H₄N₂O₂. Its structure features a six-membered ring containing two nitrogen atoms at positions 1 and 3, two carbonyl groups (C=O) at positions 2 and 4, and two carbon-hydrogen (C-H) bonds completing the ring. This specific arrangement of atoms is responsible for its unique properties and interactions within RNA. The carbonyl groups are crucial for hydrogen bonding, a key feature in the base pairing within RNA.
Uracil's Role in RNA: The RNA-Specific Base
Unlike thymine, which is exclusive to DNA, uracil is a unique feature of RNA. This difference reflects the distinct functional roles of DNA and RNA within the cell. DNA serves primarily as the long-term storage of genetic information, while RNA plays diverse roles, including protein synthesis, gene regulation, and enzymatic activity. The presence of uracil instead of thymine in RNA is believed to be a consequence of RNA's susceptibility to degradation. Uracil is more prone to hydrolysis compared to thymine, which could result in more frequent mutations. The use of uracil in RNA might therefore facilitate a faster turnover of RNA molecules, preventing the accumulation of damaged RNA.
Uracil's Base Pairing: Hydrogen Bonding and RNA Structure
Uracil's ability to form hydrogen bonds is fundamental to RNA's structure and function. In RNA, uracil specifically pairs with adenine (A) through two hydrogen bonds. This pairing is essential for maintaining the double-helical structure of some RNA molecules and also crucial for the formation of the RNA secondary structure, such as hairpin loops and stem-loop structures. This base pairing is analogous to the adenine-thymine (A-T) pairing in DNA, except that uracil replaces thymine.
Comparing Uracil to Other Bases: Highlighting the Distinctions
Comparing uracil to other pyrimidines and purines helps to highlight its unique characteristics:
Uracil vs. Thymine: The DNA-RNA Distinction
The most notable difference between uracil and thymine lies in their presence in DNA and RNA. Thymine contains a methyl group (CH3) at position 5, while uracil lacks this methyl group. This seemingly small difference has significant implications for the stability and functionality of the nucleic acids. The methyl group in thymine makes it more resistant to spontaneous deamination, a process that can lead to mutations. This heightened stability is crucial for the long-term storage of genetic information in DNA.
Uracil vs. Cytosine: Differences in Base Pairing and Reactivity
While both uracil and cytosine are pyrimidines, they have distinct base pairing partners and chemical properties. Cytosine pairs with guanine (G) through three hydrogen bonds, contributing to the stronger base pairing in DNA compared to the two hydrogen bonds formed by uracil-adenine pairs in RNA. Cytosine is also more prone to spontaneous deamination compared to uracil. This deamination converts cytosine to uracil, leading to potential mutations unless repair mechanisms are activated.
Uracil vs. Adenine and Guanine: The Purine Contrast
The most significant difference between uracil and the purine bases, adenine and guanine, is their ring structure. Adenine and guanine's double-ring structure allows for more extensive hydrogen bonding interactions than the single-ring structure of uracil. This difference leads to different stacking interactions and consequently influences the overall stability and conformation of nucleic acids.
Uracil's Role Beyond RNA: Emerging Applications
While uracil's primary role is within RNA, its unique properties are attracting attention in various fields, particularly in synthetic biology and nanotechnology. Researchers are exploring the use of uracil-modified nucleotides in gene therapy and drug delivery systems. Its ability to interact specifically with RNA offers opportunities for targeted therapeutic interventions.
SEO Optimization and Keyword Integration: A Strategic Approach
This article is carefully crafted to address numerous search queries related to uracil, including:
- Is uracil a purine or pyrimidine?
- What is the difference between uracil and thymine?
- Uracil structure and function.
- Uracil in RNA.
- The role of uracil in protein synthesis.
- Comparison of uracil, cytosine, adenine, and guanine.
These keywords and related semantic terms are naturally integrated throughout the text, improving its searchability and relevance to users seeking information on uracil's properties and classification. The use of headings, subheadings, and bold text further enhances readability and helps search engines understand the article's structure and content.
Conclusion: A Solid Understanding of Uracil's Place in Biology
In conclusion, uracil is definitively a pyrimidine. Its single-ring structure, unique role in RNA, and specific base pairing with adenine distinguish it from other bases. Understanding uracil's classification and its characteristics is critical for grasping the intricacies of nucleic acid structure and function. This comprehensive exploration of uracil provides a solid foundation for further study in molecular biology and related disciplines. The article's SEO optimization strategy ensures its discoverability for those seeking this essential information.
Latest Posts
Latest Posts
-
Which Statement Correctly Describes Magnetic Field Lines
Mar 25, 2025
-
Integration Of X 2e X 2
Mar 25, 2025
-
What Element Has 3 Protons And 4 Neutrons
Mar 25, 2025
-
What Is The Difference Between A Cell And Tissue
Mar 25, 2025
-
Integral 2xdx From 10 To 13
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Uracil A Pyrimidine Or Purine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
