Is Sodium Solid Liquid Or Gas
News Leon
Mar 20, 2025 · 5 min read
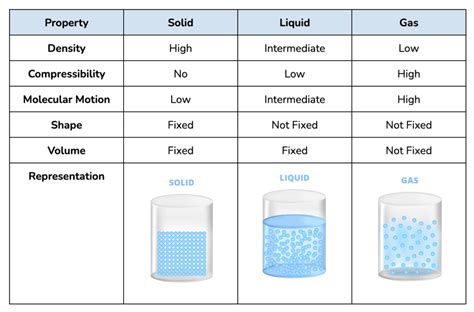
Table of Contents
Is Sodium Solid, Liquid, or Gas? Exploring the Properties of an Alkali Metal
Sodium (Na), a highly reactive alkali metal, is a fascinating element with unique properties that influence its physical state. Unlike many elements whose state is readily apparent at room temperature, understanding sodium's state requires a deeper dive into its chemical and physical characteristics. This article will comprehensively explore the question: is sodium solid, liquid, or gas? We'll delve into its melting and boiling points, its reactivity, and its applications, providing a complete picture of this essential element.
Understanding States of Matter: Solid, Liquid, and Gas
Before we dive into the specifics of sodium, let's briefly review the three fundamental states of matter:
-
Solid: In a solid, atoms or molecules are tightly packed together in a fixed, ordered arrangement. They possess strong intermolecular forces, resulting in a definite shape and volume. Solids resist changes in shape and volume.
-
Liquid: Liquids have weaker intermolecular forces than solids. Their atoms or molecules are closer together than in a gas but more loosely packed than in a solid. Liquids have a definite volume but take the shape of their container.
-
Gas: Gases have the weakest intermolecular forces. Their atoms or molecules are widely dispersed and move freely, resulting in indefinite shape and volume. Gases expand to fill the container they occupy.
The state of matter a substance exists in depends primarily on its temperature and pressure. Changes in these factors can cause transitions between solid, liquid, and gas states.
Sodium at Room Temperature and Pressure: A Solid Metal
Under standard conditions (room temperature and atmospheric pressure), sodium is a solid. Its atoms are arranged in a body-centered cubic crystal lattice, a highly ordered structure characteristic of many metals. This structure contributes to sodium's metallic properties, such as its excellent electrical and thermal conductivity. The strong metallic bonding between sodium atoms holds them firmly in place, preventing them from moving freely like in a liquid or gas.
The Melting and Boiling Points of Sodium: Transitions to Liquid and Gas
The transition of sodium from one state to another is determined by its melting and boiling points:
-
Melting Point: Sodium's melting point is relatively low at 97.8 °C (208.0 °F). This means that when heated above this temperature, the kinetic energy of its atoms overcomes the metallic bonding forces, causing the solid structure to break down and transform into a liquid.
-
Boiling Point: Sodium's boiling point is 883 °C (1621 °F). At this temperature, the kinetic energy of the sodium atoms is high enough to overcome the attractive forces entirely, allowing them to escape into the gaseous phase.
Therefore, to observe liquid sodium, you need to heat it above 97.8 °C. Observing gaseous sodium requires significantly higher temperatures, exceeding 883 °C. At these high temperatures, appropriate safety precautions are absolutely essential due to sodium's high reactivity.
The Reactivity of Sodium: A Key Consideration
Sodium's high reactivity plays a crucial role in its handling and observation. It reacts violently with water, producing hydrogen gas and sodium hydroxide, a highly caustic substance. This reaction releases a significant amount of heat, sometimes igniting the hydrogen gas. This is why sodium is always stored under an inert atmosphere (like argon or kerosene) to prevent it from reacting with oxygen or moisture in the air. This reactivity significantly limits opportunities for casual observation of liquid or gaseous sodium.
Applications of Sodium: From Industry to Medicine
Despite its reactivity, sodium is a versatile element with numerous applications across various industries:
-
Sodium-Vapor Lamps: Sodium in its gaseous state is used in sodium-vapor lamps, which produce a characteristic yellow light. The intense light emission from excited sodium atoms makes them exceptionally efficient for street lighting and other outdoor illumination applications. The lamps operate at high temperatures to vaporize the sodium.
-
Chemical Industry: Sodium is used as a reducing agent in various chemical processes, such as the production of titanium and other metals. Its strong reducing properties allow it to extract metals from their ores.
-
Nuclear Reactors: Liquid sodium is employed as a coolant in some nuclear reactors because of its excellent heat transfer properties. Its relatively low melting point and high boiling point make it well-suited for this purpose, although the potential for sodium-water reactions necessitates rigorous safety protocols.
-
Organic Chemistry: Sodium is crucial in various organic synthesis reactions. For example, it's used in the preparation of sodium alkoxides, which are vital reagents in organic chemistry.
Safety Precautions When Handling Sodium
Due to its high reactivity, sodium must be handled with extreme care. Direct contact with skin or eyes can cause severe burns. Inhaling sodium dust can be harmful to the respiratory system. Always follow these safety precautions:
- Use appropriate personal protective equipment (PPE): This includes safety glasses, gloves, and a lab coat.
- Work in a well-ventilated area: To prevent accumulation of hydrogen gas in case of accidental contact with water.
- Store sodium under an inert atmosphere: To prevent oxidation and reaction with moisture.
- Never dispose of sodium in water: This can cause a violent reaction and potential fire hazard. Proper disposal methods should always be followed.
Conclusion: Sodium's State in Different Contexts
In summary, under standard conditions, sodium exists as a solid. However, its ability to transition to liquid and gaseous states is well-defined by its melting and boiling points. While observing liquid or gaseous sodium requires specialized conditions and safety precautions due to its high reactivity, understanding its diverse physical states and applications is crucial for appreciating its importance in various fields, from street lighting to nuclear reactors and chemical synthesis. The inherent reactivity of sodium necessitates cautious handling and adherence to strict safety protocols in all scenarios. Understanding these factors is paramount for safe and responsible handling of this powerful element.
Latest Posts
Latest Posts
-
Into How Many Time Zones Is Earth Divided
Mar 20, 2025
-
During Which Stage Of Cell Cycle Does Dna Replication Occur
Mar 20, 2025
-
Copper And Aluminum Are Being Considered For A High Voltage Transmission
Mar 20, 2025
-
Physical Properties Of A Covalent Compound
Mar 20, 2025
-
In Figure 1 A 3 50 G Bullet
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
