Is Nh3 A Lewis Acid Or Base
News Leon
Mar 19, 2025 · 5 min read
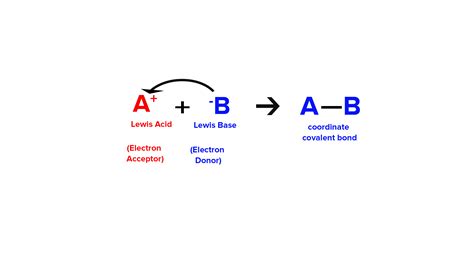
Table of Contents
Is NH₃ a Lewis Acid or Base? Understanding the Nature of Ammonia
Ammonia (NH₃), a simple yet crucial molecule in chemistry, often sparks debate regarding its classification as a Lewis acid or base. While its Brønsted-Lowry behavior is relatively straightforward, understanding its Lewis acid-base properties requires a deeper dive into the concepts of electron donation and acceptance. This comprehensive article will explore the nature of ammonia, definitively answering whether it's a Lewis acid or base, and examining the nuanced details that underpin this classification.
Understanding Lewis Acid-Base Theory
Before delving into ammonia's behavior, let's revisit the fundamental principles of the Lewis acid-base theory. Unlike the Brønsted-Lowry theory, which focuses on proton (H⁺) transfer, the Lewis theory centers on the donation and acceptance of electron pairs.
-
Lewis Acid: A Lewis acid is defined as an electron-pair acceptor. It possesses an empty orbital that can accommodate a pair of electrons. Examples include metal cations (like Al³⁺) and molecules with incomplete octets (like BF₃).
-
Lewis Base: A Lewis base is defined as an electron-pair donor. It possesses a lone pair of electrons that it can share with a Lewis acid. Examples include ammonia (NH₃), water (H₂O), and hydroxide ions (OH⁻).
The key difference lies in the focus: proton transfer versus electron pair donation/acceptance. This distinction is crucial when considering molecules like ammonia.
Ammonia's Structure and Electron Configuration
Ammonia's molecular structure is crucial in determining its Lewis acid-base behavior. It has a trigonal pyramidal geometry, with the nitrogen atom at the apex and three hydrogen atoms at the base. The nitrogen atom is sp³ hybridized. This hybridization leads to a lone pair of electrons on the nitrogen atom. This lone pair is the cornerstone of ammonia's Lewis base properties.
The nitrogen atom in ammonia has five valence electrons. Three of these electrons are used to form covalent bonds with the three hydrogen atoms. The remaining two electrons constitute the lone pair. It's this lone pair that makes ammonia a potent Lewis base.
Ammonia as a Lewis Base: Evidence and Examples
The presence of the lone pair directly leads to ammonia's classification as a Lewis base. The lone pair of electrons on the nitrogen atom is readily available to be donated to a Lewis acid, forming a coordinate covalent bond (also known as a dative bond).
Several examples illustrate ammonia's Lewis base behavior:
-
Reaction with Boron Trifluoride (BF₃): BF₃ is a classic example of a Lewis acid, possessing an incomplete octet. Ammonia readily reacts with BF₃, donating its lone pair to the boron atom, forming a stable adduct, H₃N-BF₃. This reaction clearly demonstrates ammonia's ability to act as an electron-pair donor, fulfilling the definition of a Lewis base.
-
Formation of Amine Complexes: Ammonia forms complexes with various metal ions, acting as a ligand. These complexes are formed due to the donation of the lone pair on nitrogen to the metal ion's empty orbitals. For example, the formation of tetraamminecopper(II) ion, [Cu(NH₃)₄]²⁺, showcases ammonia's ability to coordinate with a transition metal ion through its lone pair.
-
Reactions with Protic Acids: While ammonia's Brønsted-Lowry behavior is well-known (it acts as a base by accepting a proton), this behavior is a subset of its Lewis base behavior. Accepting a proton (H⁺) is essentially accepting an electron-deficient species (H⁺ is essentially a bare proton).
Can Ammonia Act as a Lewis Acid?
While ammonia's primary role is as a Lewis base, there are extremely rare circumstances where it could potentially exhibit weak Lewis acidic behavior. This requires specific, highly unusual conditions and is not its characteristic behavior. These cases generally involve interactions with exceptionally strong Lewis bases under extremely specific conditions. The nitrogen atom's ability to accept electron density is significantly less pronounced than its capacity to donate it.
Comparing Brønsted-Lowry and Lewis Acid-Base Behavior in Ammonia
It's important to differentiate between ammonia's Brønsted-Lowry and Lewis acid-base properties:
-
Brønsted-Lowry: Ammonia acts as a Brønsted-Lowry base by accepting a proton (H⁺) from a Brønsted-Lowry acid. This is a common and well-established property. The reaction of ammonia with hydrochloric acid (HCl) to form ammonium chloride (NH₄Cl) is a prime example.
-
Lewis: Ammonia acts as a Lewis base by donating its lone pair of electrons to a Lewis acid. This broader definition encompasses the Brønsted-Lowry behavior, as proton acceptance is a specific instance of electron pair donation.
Practical Applications of Ammonia's Lewis Basicity
Ammonia's Lewis basicity is exploited extensively in various applications:
-
Synthesis of Nitrogen-Containing Compounds: Its ability to donate electrons is critical in the synthesis of various nitrogen-containing compounds, including amines, amides, and nitriles.
-
Catalysis: Ammonia acts as a ligand in many catalytic systems, influencing the reactivity and selectivity of catalysts.
-
Coordination Chemistry: As a ligand, ammonia plays a crucial role in coordination chemistry, forming complexes with numerous transition metal ions. This has applications in areas like material science and analytical chemistry.
-
Agriculture: In agriculture, ammonia is used as a nitrogen source in fertilizers, providing plants with the essential nitrogen needed for growth. This indirectly relies on its ability to react and form stable compounds.
Conclusion: Ammonia as a Predominantly Lewis Base
In conclusion, ammonia (NH₃) is unequivocally classified as a Lewis base. Its lone pair of electrons on the nitrogen atom makes it a powerful electron-pair donor, readily reacting with Lewis acids to form coordinate covalent bonds. While exceptionally rare circumstances might suggest extremely weak Lewis acidic behavior, this is not its characteristic property. Understanding ammonia's Lewis base nature is crucial for comprehending its extensive reactivity and diverse applications across various fields of chemistry and beyond. Its dominant role as a Lewis base underlies its significance in many chemical processes and industrial applications. The presence of the lone pair is the defining factor, overshadowing any negligible Lewis acidic tendencies. Therefore, labeling ammonia primarily as a Lewis base is accurate and reflective of its most prevalent behavior.
Latest Posts
Latest Posts
-
The Figure Shows A Conical Pendulum In Which The Bob
Mar 20, 2025
-
What Is The Heart Of A Computer
Mar 20, 2025
-
Which Statement Is Not Always True For A Parallelogram
Mar 20, 2025
-
What Percent Of 40 Is 16
Mar 20, 2025
-
Which Of The Following Has The Higher Energy
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Nh3 A Lewis Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
