Is Nh2 Electron Donating Or Withdrawing
News Leon
Apr 03, 2025 · 6 min read
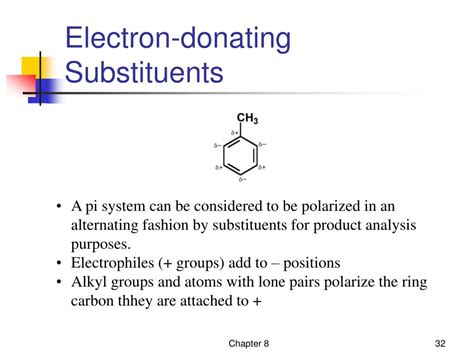
Table of Contents
- Is Nh2 Electron Donating Or Withdrawing
- Table of Contents
- Is NH2 Electron Donating or Withdrawing? A Comprehensive Look at the Amino Group
- Understanding Electron Donation and Withdrawal
- The Dual Nature of the Amino Group: Inductive vs. Resonance Effects
- Inductive Effect
- Resonance Effect
- The Context Matters: When NH2 Donates and When it Withdraws
- NH2 as an Electron-Donating Group
- NH2 as an Electron-Withdrawing Group (relatively weak)
- Consequences of NH2's Electronic Effects
- Influence on Acidity and Basicity
- Impact on Reaction Rates
- Spectroscopic Properties
- Examples Illustrating the Dual Nature
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Is NH2 Electron Donating or Withdrawing? A Comprehensive Look at the Amino Group
The amino group (-NH2) is a ubiquitous functional group in organic chemistry and biochemistry, found in amino acids, amines, and amides. Understanding its electronic properties is crucial for predicting reactivity and understanding the behavior of molecules containing this group. The central question, "Is NH2 electron donating or withdrawing?", isn't a simple yes or no answer. Its behavior is nuanced and depends heavily on the context. This article will delve deep into the electronic nature of the NH2 group, examining its inductive and resonance effects, and exploring how these effects influence its behavior in different chemical environments.
Understanding Electron Donation and Withdrawal
Before we dissect the amino group's properties, let's establish a clear understanding of electron donation and withdrawal. These terms describe how a substituent affects the electron density of a molecule, particularly at a nearby reactive center.
-
Electron-donating groups (EDGs): These groups increase the electron density at a nearby atom or group. They are often characterized by the presence of lone pairs of electrons or multiple bonds that can readily donate electron density through inductive or resonance effects.
-
Electron-withdrawing groups (EWGs): These groups decrease the electron density at a nearby atom or group. They often contain electronegative atoms (like oxygen, fluorine, chlorine, etc.) that attract electrons towards themselves.
The Dual Nature of the Amino Group: Inductive vs. Resonance Effects
The amino group's behavior is complex because it exhibits both inductive and resonance effects, which can sometimes oppose each other.
Inductive Effect
The inductive effect is a permanent effect that arises from the electronegativity difference between atoms in a molecule. Nitrogen is more electronegative than carbon and hydrogen. Therefore, the nitrogen atom in the NH2 group tends to pull electron density away from the carbon atom to which it's attached. This is an electron-withdrawing inductive effect.
Think of it like this: Nitrogen is like a tiny vacuum cleaner, subtly sucking electron density from its surroundings.
Resonance Effect
The resonance effect, however, presents a different picture. The nitrogen atom in the NH2 group possesses a lone pair of electrons which can participate in resonance with adjacent pi systems (like those found in benzene rings or carbonyl groups). This lone pair can delocalize into the pi system, thereby increasing the electron density in the conjugated system. This is a powerful electron-donating resonance effect.
Visualize this: The lone pair on nitrogen acts like a water hose, spraying electron density into a nearby pi system.
The Context Matters: When NH2 Donates and When it Withdraws
The overall effect of the NH2 group (electron-donating or electron-withdrawing) is highly dependent on the context, particularly whether resonance is possible.
NH2 as an Electron-Donating Group
The amino group acts as a strong electron-donating group when:
-
Resonance is possible: If the NH2 group is directly attached to a conjugated pi system (like a benzene ring or a carbonyl group), the resonance effect dominates. The lone pair on nitrogen delocalizes into the pi system, making the NH2 group a strong activator in electrophilic aromatic substitution reactions. For example, aniline (C6H5NH2) is significantly more reactive than benzene towards electrophilic substitution.
-
Through Conjugation: The electron-donating effect through resonance significantly outweighs the electron-withdrawing inductive effect when the NH2 group is conjugated.
-
Aromatic Systems: In aromatic compounds, the resonance effect is exceptionally pronounced, making the amino group a powerful activating group.
NH2 as an Electron-Withdrawing Group (relatively weak)
The amino group acts as a relatively weak electron-withdrawing group when:
-
Resonance is not possible: In the absence of a conjugated pi system, the inductive effect becomes more prominent. While still weak compared to strong EWGs, the electronegativity of nitrogen slightly withdraws electron density. The impact is considerably less than when resonance is involved.
-
Saturated Systems: In saturated systems (those lacking pi bonds), the inductive effect is the primary player. In these cases, the electron-withdrawing inductive effect is noticeable, but not as powerful as the electron-donating resonance effect in conjugated systems.
Consequences of NH2's Electronic Effects
The electronic properties of the amino group have profound consequences on the reactivity and properties of molecules containing it.
Influence on Acidity and Basicity
-
Basicity: The lone pair on nitrogen makes the amino group a good base. It readily accepts protons, forming ammonium ions (NH3+). The basicity of the NH2 group can be affected by the presence of electron-donating or electron-withdrawing groups on the nitrogen atom or nearby.
-
Acidity: The NH2 group is not acidic; it's a relatively poor proton donor.
Impact on Reaction Rates
-
Electrophilic Aromatic Substitution: In aromatic systems, the NH2 group's strong electron-donating resonance effect activates the ring towards electrophilic attack, leading to faster reaction rates.
-
Nucleophilic Reactions: The presence of a lone pair also makes the amino group a good nucleophile, which can participate in various nucleophilic substitution reactions.
Spectroscopic Properties
The electronic effects of the NH2 group can also be observed in spectroscopic properties:
-
Infrared (IR) Spectroscopy: The NH stretching vibrations appear in the IR spectrum, often in the range of 3300-3500 cm⁻¹.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: The chemical shift of protons attached to nitrogen or the carbon atom adjacent to the amino group is influenced by the electronic environment.
Examples Illustrating the Dual Nature
Let's consider a few examples to illustrate the dual nature of the NH2 group:
-
Aniline (C6H5NH2): The NH2 group is directly attached to a benzene ring, allowing for significant resonance. The resonance effect overwhelms the inductive effect, making aniline strongly activated towards electrophilic aromatic substitution.
-
Methylamine (CH3NH2): Here, the NH2 group is attached to a saturated carbon atom. Resonance is not possible, so the weaker electron-withdrawing inductive effect is more noticeable.
-
Acetamide (CH3CONH2): The NH2 group is attached to a carbonyl group, allowing for resonance. However, the resonance effect is less dominant compared to aniline because the carbonyl group itself is an electron-withdrawing group.
Conclusion
The question of whether the NH2 group is electron-donating or electron-withdrawing doesn't have a simple answer. Its behavior is a complex interplay of inductive and resonance effects. The dominant effect depends entirely on the molecular environment. In the presence of conjugated pi systems, the powerful electron-donating resonance effect prevails, making NH2 a strong activator. In saturated systems without conjugation, the weaker electron-withdrawing inductive effect is more noticeable. Understanding this dual nature is critical for predicting the reactivity and properties of molecules containing the amino group, a functional group of immense importance in organic chemistry and biochemistry. By considering both the inductive and resonance effects and the context in which the NH2 group is found, one can accurately predict its influence on the overall electronic properties of the molecule.
Latest Posts
Latest Posts
-
Which Best Describes The Law Of Independent Assortment
Apr 06, 2025
-
You Should Always Assign The Needs Met Rating Before
Apr 06, 2025
-
Which Is The Most Densely Populated Continent
Apr 06, 2025
-
Average Velocity On Velocity Time Graph
Apr 06, 2025
-
The First Scientist To Observe Cells With A Microscope Was
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about Is Nh2 Electron Donating Or Withdrawing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
