Is Fluorine A Cation Or Anion
News Leon
Mar 20, 2025 · 5 min read
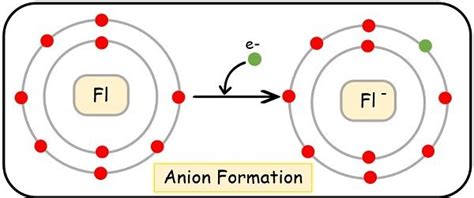
Table of Contents
Is Fluorine a Cation or an Anion? Understanding its Ionic Nature
Fluorine, the most electronegative element on the periodic table, plays a crucial role in various chemical processes. A common question that arises when studying its chemical behavior is whether it acts as a cation (positively charged ion) or an anion (negatively charged ion). The answer, however, is straightforward and fundamental to understanding its reactivity. Fluorine almost exclusively exists as an anion (F⁻). This article will delve into the reasons behind this, explore its ionic nature, and discuss the implications of its behavior in different chemical contexts.
Understanding Electronegativity and Ion Formation
Before delving into the specifics of fluorine's ionic nature, it's crucial to understand the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. The higher the electronegativity value, the stronger the atom's pull on shared electrons. Fluorine boasts the highest electronegativity of all elements. This exceptionally high electronegativity dictates its behavior in chemical reactions and fundamentally determines whether it will gain or lose electrons to form an ion.
Fluorine's High Electronegativity: The Driving Force
Fluorine's high electronegativity stems from its small atomic size and the strong nuclear charge experienced by its outermost electrons. These outermost electrons are held tightly by the nucleus, making it energetically favorable for fluorine to gain an electron rather than lose one. Losing an electron would require overcoming the strong attractive force of the nucleus, demanding a significant amount of energy. Gaining an electron, however, results in a stable electron configuration, completing its outermost shell and achieving a noble gas configuration (like neon). This energetically favorable state drives fluorine to readily accept an electron, forming a stable anion.
The Octet Rule and Fluorine's Anionic Nature
The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell. Fluorine, with seven electrons in its outermost shell, needs only one more electron to achieve a stable octet. By gaining an electron, it readily achieves this stable configuration, forming the fluoride ion (F⁻), thereby fulfilling the octet rule. This explains the overwhelming preference of fluorine to form anions rather than cations.
Why Fluorine Doesn't Form Cations
The energy required to remove an electron from fluorine to form a cation (F⁺) is incredibly high. This is because the strong nuclear charge tightly binds the valence electrons. The energy cost associated with ionization far outweighs the energy gained from forming a cation. Consequently, the formation of a fluorine cation is highly improbable under normal chemical conditions. In essence, the energy barrier for ionization is too high for fluorine to overcome. This is a stark contrast to elements with lower electronegativities, which may readily lose electrons to form cations.
Exceptional Circumstances and Theoretical Considerations
While highly improbable, there might be some exceptional circumstances under extreme conditions (like in a plasma or highly energetic environment) where a fluorine cation could theoretically be formed. However, these are far from typical chemical reactions and are not relevant to the majority of chemical scenarios where fluorine is involved. The vast majority of chemical reactions involving fluorine see it forming the stable fluoride anion (F⁻).
The Fluoride Ion (F⁻): Properties and Reactions
The fluoride ion (F⁻), formed by fluorine gaining one electron, is a relatively small and highly reactive anion. Its small size leads to a high charge density, which contributes to its strong interactions with other ions and molecules. This high charge density is directly responsible for the strong ionic bonds formed by fluoride with various cations.
Reactions of the Fluoride Ion
The fluoride ion participates in a variety of chemical reactions, primarily due to its high reactivity and its ability to form strong ionic bonds. Some important examples include:
-
Formation of ionic compounds: Fluoride readily forms ionic compounds with various metals. Examples include sodium fluoride (NaF), calcium fluoride (CaF₂), and aluminum fluoride (AlF₃). These compounds are often used in various applications, ranging from dental care to industrial processes.
-
Reactions with acids: Fluoride can react with acids to form hydrofluoric acid (HF), a weak acid that is highly corrosive. This acid is important in various industrial applications, particularly in the etching of glass.
-
Complex formation: The fluoride ion can act as a ligand, forming complexes with various metal ions. These complexes often exhibit unique properties and are important in various chemical and biological systems.
-
Reactions in biological systems: Fluoride plays a critical role in biological systems, particularly in the prevention of dental caries (tooth decay). It strengthens tooth enamel by forming fluorapatite, a more resistant mineral compared to the naturally occurring hydroxyapatite.
Fluorine in Organic Chemistry
Fluorine's influence extends significantly into organic chemistry. The incorporation of fluorine atoms into organic molecules, a process known as fluorination, often leads to significant changes in the physical and chemical properties of the resulting compounds. These changes can be exploited for various applications.
Applications of Fluorinated Organic Compounds
Fluorinated organic compounds have widespread applications in various fields:
-
Pharmaceuticals: Fluorine substitution can enhance the potency, metabolic stability, and bioavailability of pharmaceuticals. Many commercially available drugs contain fluorine atoms.
-
Materials Science: Fluorinated polymers, such as Teflon (polytetrafluoroethylene), exhibit unique properties like high thermal stability, chemical inertness, and low friction. These properties lead to their use in various applications including non-stick cookware and high-performance materials.
-
Agrochemicals: Fluorine substitution can improve the efficacy and persistence of agrochemicals, but environmental concerns surrounding the use of fluorinated compounds in this area warrant careful consideration.
Conclusion: The Definitive Anionic Nature of Fluorine
In conclusion, fluorine overwhelmingly exists as an anion (F⁻) due to its exceptionally high electronegativity. Its strong tendency to gain an electron to achieve a stable octet configuration makes the formation of a fluoride anion highly favorable. While theoretical considerations allow for the possibility of a fluorine cation under extreme conditions, this is not relevant under typical chemical circumstances. Understanding fluorine's anionic nature is fundamental to grasping its chemical behavior and its significance in various fields, from inorganic chemistry and materials science to organic chemistry and biology. The fluoride ion's properties and reactivity have led to its widespread use in numerous applications, highlighting its importance in modern science and technology. Further research continues to explore the diverse applications of fluorine and its compounds, pushing the boundaries of innovation and technological advancements.
Latest Posts
Latest Posts
-
Oxidation Number Of N In Nh3
Mar 20, 2025
-
What Should We Do To Make Friends With The Wind
Mar 20, 2025
-
What Is The Percentage Of 20 Out Of 30
Mar 20, 2025
-
96 Is What Percent Of 150
Mar 20, 2025
-
The Most Abundant Cation In Intracellular Fluid Is Sodium
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Fluorine A Cation Or Anion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
