Is Dissolution Of Salt In Water A Physical Change
News Leon
Mar 15, 2025 · 5 min read
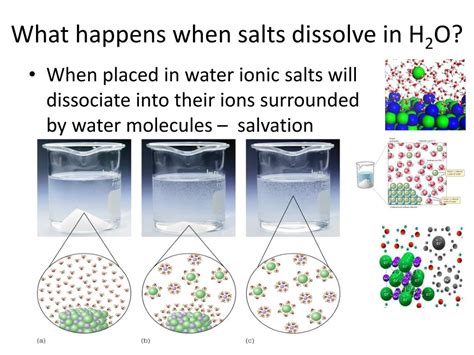
Table of Contents
- Is Dissolution Of Salt In Water A Physical Change
- Table of Contents
- Is Dissolving Salt in Water a Physical Change? A Deep Dive into States of Matter and Solutions
- Understanding Physical and Chemical Changes
- Physical Changes: A Matter of Form, Not Substance
- Chemical Changes: A Transformation of Substance
- The Case of Salt Dissolving in Water: A Closer Look
- The Process of Dissolution: A Microscopic View
- Why it's Not a Chemical Change
- Distinguishing Physical Changes from Chemical Changes in Solution Formation
- The Importance of Hydration and Ion-Dipole Interactions
- Debunking Common Misconceptions
- Applications and Real-World Examples
- Conclusion: A Physical Phenomenon with Far-Reaching Consequences
- Latest Posts
- Latest Posts
- Related Post
Is Dissolving Salt in Water a Physical Change? A Deep Dive into States of Matter and Solutions
The question of whether dissolving salt in water is a physical or chemical change is a common one, frequently arising in chemistry classes and sparking debate among science enthusiasts. While seemingly simple, the answer requires a nuanced understanding of the definitions of physical and chemical changes, the properties of matter, and the nature of solutions. This comprehensive exploration delves into the intricacies of this process, providing a detailed analysis supported by scientific principles.
Understanding Physical and Chemical Changes
Before diving into the specifics of salt dissolving in water, let's clarify the fundamental difference between physical and chemical changes.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same, even if its physical state (solid, liquid, gas) or shape changes. Examples include melting ice (solid water becoming liquid water), boiling water (liquid water becoming gaseous water), crushing a can, or dissolving sugar in water. Crucially, no new substance is formed. The original substance can be recovered through physical means (e.g., evaporating the water to recover the dissolved sugar).
Chemical Changes: A Transformation of Substance
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. These changes often involve breaking and forming chemical bonds. Evidence of a chemical change includes a color change, the formation of a precipitate (solid), the evolution of a gas, or a change in temperature. Examples include burning wood, rusting iron, or baking a cake. The original substances cannot be easily recovered in their original form.
The Case of Salt Dissolving in Water: A Closer Look
When we dissolve table salt (sodium chloride, NaCl) in water, the salt crystals appear to disappear, creating a homogeneous mixture – a solution. Does this constitute a physical or chemical change?
The answer is: it's primarily a physical change.
The Process of Dissolution: A Microscopic View
At the microscopic level, the process involves the following:
-
Water molecules, polar in nature, interact with the ions in the salt crystal. Water molecules have a slightly positive end (hydrogen atoms) and a slightly negative end (oxygen atom). These opposite charges attract the positively charged sodium ions (Na⁺) and the negatively charged chloride ions (Cl⁻) in the salt crystal.
-
Hydration: The water molecules surround and encapsulate the individual sodium and chloride ions, a process called hydration. This weakens the electrostatic forces holding the ions together in the crystal lattice.
-
Dissociation: The hydrated ions become separated from the crystal and disperse throughout the water, forming a solution. The ions retain their chemical identity; they are still sodium and chloride ions.
Why it's Not a Chemical Change
No new chemical substances are formed when salt dissolves in water. The sodium and chloride ions remain sodium and chloride ions. We can easily recover the salt by evaporating the water. This reversibility is a strong indicator of a physical change. The chemical bonds within the NaCl molecules are not broken during the dissolution process; instead, the interionic forces within the crystal are overcome.
Distinguishing Physical Changes from Chemical Changes in Solution Formation
It's important to note that some solutions do involve chemical changes. For example, dissolving certain metals in acids can result in a chemical reaction, producing hydrogen gas and a new salt. However, this is not the case with dissolving salt in water.
Here's a table summarizing the key differences to help distinguish physical changes from chemical changes during solution formation:
| Feature | Physical Change (Salt in Water) | Chemical Change |
|---|---|---|
| Substance | Original substances retained | New substances formed |
| Chemical Bonds | Bonds within solute molecules not broken | Bonds broken and reformed |
| Reversibility | Easily reversible (evaporation) | Generally irreversible |
| Energy Change | Small energy change (endothermic) | Significant energy change (exothermic/endothermic) |
| Properties | Chemical properties unchanged | Chemical properties significantly altered |
The Importance of Hydration and Ion-Dipole Interactions
The process of hydration is crucial to understanding why salt dissolves in water. The strong ion-dipole interactions between the polar water molecules and the charged ions provide the energy needed to overcome the lattice energy of the salt crystal. This energy exchange is relatively small, supporting the classification of the process as a physical change.
Debunking Common Misconceptions
Some people might argue that a change has occurred since the salt is no longer visible as a solid crystal. However, the change is only a physical state transformation; the chemical identity of the sodium and chloride ions remains unchanged.
Another misconception arises from the perceived "mixing" of two substances. While a mixture is created, the fundamental chemical nature of the salt remains intact. The dissolution is primarily a dispersion of ions in the solvent, not a chemical reaction.
Applications and Real-World Examples
The dissolution of salt in water is a fundamental process with widespread applications:
- Electrolyte Solutions: Saltwater is a common electrolyte solution used in batteries and other electrochemical devices. The dissolved ions conduct electricity.
- Food Preservation: Salt has been used for centuries to preserve food by creating a hypertonic environment that inhibits microbial growth.
- Medical Applications: Saline solutions (saltwater) are essential in various medical procedures, such as intravenous fluid administration.
- Industrial Processes: Saltwater is used in many industrial processes, including water softening and chemical manufacturing.
Conclusion: A Physical Phenomenon with Far-Reaching Consequences
In conclusion, dissolving salt in water is primarily a physical change. While it involves a change in physical state and appearance, the chemical composition of the salt remains unaltered. The process of dissolution is driven by ion-dipole interactions between water molecules and the salt ions, resulting in the hydration and dispersion of ions. Understanding this fundamental process is crucial in various fields, highlighting its importance beyond the classroom. Although seemingly simple, the intricacies of dissolving salt in water demonstrate the power of intermolecular forces and the fascinating interplay of matter at the microscopic level. The reversibility of the process, the absence of new chemical substances, and the relatively small energy involved clearly point to a physical, rather than a chemical transformation. This knowledge is essential for comprehending a wide range of chemical and physical phenomena, from biological processes to industrial applications.
Latest Posts
Latest Posts
-
8 5 On A Number Line
Mar 16, 2025
-
What Is 1000 Days In Years
Mar 16, 2025
-
5 Examples Of Combustion In Everyday Life
Mar 16, 2025
-
Which Of The Following Statements Is True About Alzheimers Disease
Mar 16, 2025
-
The Nucleus Is Surrounded By The
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Is Dissolution Of Salt In Water A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
