Is Brass A Homogeneous Or Heterogeneous Mixture
News Leon
Mar 24, 2025 · 5 min read
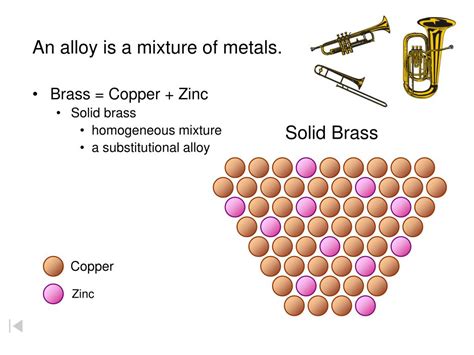
Table of Contents
Is Brass a Homogeneous or Heterogeneous Mixture? A Deep Dive into Material Science
Brass, a visually appealing and widely used alloy, often sparks curiosity regarding its classification as a mixture. Is it homogeneous, meaning its composition is uniform throughout, or heterogeneous, exhibiting variations in its constituents? The answer, while seemingly straightforward, delves into the fascinating world of material science and the intricacies of alloy formation. This comprehensive article will unravel the complexities of brass's composition, explore the criteria for classifying mixtures, and ultimately determine its true nature.
Understanding Homogeneous and Heterogeneous Mixtures
Before classifying brass, it's crucial to establish a clear understanding of the terms "homogeneous" and "heterogeneous."
Homogeneous Mixtures: Uniformity at the Microscopic Level
A homogeneous mixture is characterized by a uniform composition throughout its entire volume. This means that no matter where you take a sample from the mixture, its composition will remain consistent at the macroscopic and microscopic levels. Solutions, like saltwater or sugar dissolved in water, are prime examples. Even under magnification, the components are indistinguishable, forming a single phase.
Heterogeneous Mixtures: Visible Variations in Composition
In contrast, a heterogeneous mixture demonstrates visibly distinct regions with varying compositions. The different components remain separate and can be easily identified. Examples include sand and water, oil and water, or a salad. Heterogeneous mixtures often exhibit multiple phases, easily observable even without magnification.
The Composition of Brass: A Closer Look
Brass is an alloy primarily composed of copper (Cu) and zinc (Zn). The proportions of these two elements can vary significantly, leading to different types of brass with distinct properties. This variability is a key factor in determining its classification.
The Role of Copper and Zinc
Copper, a reddish-brown metal known for its excellent conductivity, forms the base of most brass alloys. Zinc, a bluish-white metal, is added to enhance the mechanical properties of copper, increasing its strength, hardness, and machinability. The specific ratio of copper to zinc dictates the final properties of the resulting brass, such as its color, ductility, and corrosion resistance.
Variations in Brass Composition: Creating Diverse Properties
The versatility of brass stems from the wide range of possible copper-zinc ratios. Some common brass types include:
- Cartridge brass (70% Cu, 30% Zn): Known for its high ductility, making it ideal for drawing into wires and tubes.
- Red brass (85% Cu, 15% Zn): Exhibits excellent corrosion resistance and is often used in plumbing fixtures.
- Yellow brass (65% Cu, 35% Zn): A stronger and more durable brass, commonly employed in decorative applications.
The ability to tailor the properties of brass by adjusting the copper-zinc ratio demonstrates the fundamental principle of alloying—combining metals to create materials with enhanced characteristics.
Classifying Brass: Homogeneous or Heterogeneous?
Given the detailed understanding of both homogeneous and heterogeneous mixtures and the composition of brass, we can now address the central question. At the macroscopic level, brass is considered a homogeneous mixture. This is because the copper and zinc atoms are distributed evenly throughout the alloy at a scale visible to the naked eye or even under low magnification. The different atoms are not visibly separated into distinct regions or phases.
However, it's important to consider the microscopic level. At an extremely high magnification, using techniques like electron microscopy, a more complex picture emerges. While the distribution of copper and zinc atoms is generally uniform, some minor variations may exist due to the solidification process during brass manufacturing. This could lead to minute variations in composition in certain microscopic regions. Despite this microscopic heterogeneity, the macroscopic uniformity makes the overall classification as a homogeneous mixture appropriate for most practical applications.
Factors Influencing Homogeneity in Brass
Several factors play a crucial role in achieving a high degree of homogeneity in brass:
Manufacturing Processes: Crucial for Uniformity
The manufacturing process significantly influences the final homogeneity of the brass. Techniques like casting, rolling, and drawing can affect the distribution of the constituent elements. Careful control over temperature, pressure, and processing time is essential to achieve uniform mixing.
Cooling Rates: Impacts Atomic Arrangement
The rate at which molten brass cools also affects the homogeneity. Rapid cooling might lead to slight inconsistencies in the distribution of zinc and copper atoms, while slow cooling generally promotes a more uniform structure.
Alloying Additives: Modifying Properties and Homogeneity
Some brass alloys include small amounts of other elements, such as lead (Pb), tin (Sn), or aluminum (Al), to further enhance specific properties. These additives, when properly incorporated, do not drastically alter the homogeneous nature of the alloy, but their distribution should be uniformly controlled to avoid any significant heterogeneous regions.
Practical Implications of Brass's Homogeneity
The homogeneous nature of brass has several important practical implications:
Predictable Material Properties: Ensuring Consistent Performance
The consistent composition throughout the brass alloy guarantees predictable material properties. This makes it highly suitable for engineering applications requiring reliable performance, such as in plumbing, electrical components, and musical instruments.
Easier Machining and Fabrication: Simplified Manufacturing
The uniform structure of brass makes it relatively easy to machine and fabricate. This simplifies the manufacturing process and allows for high-precision shaping.
Consistent Appearance: Ideal for Decorative Applications
The homogeneity also results in a consistent visual appearance, making brass a popular choice for decorative purposes. The uniform color and texture make it aesthetically pleasing.
Conclusion: A Homogeneous Alloy with Microscopic Nuances
In conclusion, brass is predominantly considered a homogeneous mixture. While extremely high magnification might reveal minor microscopic variations in the distribution of copper and zinc atoms, these variations are generally insignificant at the macroscopic level. The even distribution of its constituents results in consistent and predictable material properties, making it a valuable material in a wide range of applications. The homogeneity of brass is a direct consequence of its manufacturing process and the inherent nature of its constituents' atomic-level mixing. Therefore, the practical classification of brass remains firmly in the homogeneous category. Understanding the nuances of its composition and the implications of its homogeneity is essential for anyone working with or studying this versatile and widely used alloy.
Latest Posts
Latest Posts
-
A Group Of Cells With Similar Structure And Function
Mar 29, 2025
-
Integrate Sqrt A 2 X 2
Mar 29, 2025
-
How Many Different Phone Numbers Are Possible
Mar 29, 2025
-
What Day Will It Be In 150 Days
Mar 29, 2025
-
Black Fur In Mice Is Dominant To Brown Fur
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Brass A Homogeneous Or Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
