Is Boron A Solid Liquid Or Gas
News Leon
Mar 16, 2025 · 5 min read
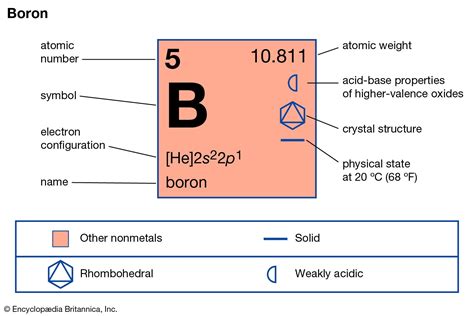
Table of Contents
Is Boron a Solid, Liquid, or Gas? Understanding Boron's Properties
Boron, a fascinating metalloid element, often sparks curiosity about its physical state. Unlike many elements, classifying boron as solely a solid, liquid, or gas is an oversimplification. Its behavior is more nuanced, dictated by temperature and pressure. This comprehensive guide will delve into the intricacies of boron's existence, exploring its unique properties and clarifying its state of matter under various conditions. We'll explore its crystalline structure, its melting and boiling points, and the conditions under which it might exhibit different behaviors.
Understanding States of Matter
Before diving into the specifics of boron, let's briefly revisit the three fundamental states of matter: solid, liquid, and gas.
- Solid: In a solid state, atoms or molecules are tightly packed in a highly ordered arrangement, exhibiting a fixed shape and volume. Strong intermolecular forces maintain this rigid structure.
- Liquid: Liquids have a definite volume but no fixed shape. Their atoms or molecules are less tightly packed than in solids, allowing them to flow and adapt to the shape of their container.
- Gas: Gases have neither a fixed shape nor a fixed volume. Their atoms or molecules are widely dispersed, moving freely and independently, leading to compressibility and expansion.
Boron: A Metalloid with Unique Characteristics
Boron, with its atomic number 5 and symbol B, sits on the borderline between metals and nonmetals, placing it in the metalloid category. This classification highlights its unusual combination of properties, contributing to its complex behavior regarding its state of matter. Metalloids exhibit characteristics of both metals and nonmetals, often leading to unpredictable physical and chemical behaviors.
Boron's Crystalline Structure: The Foundation of its Solid State
At standard temperature and pressure (STP), boron exists as a solid. Its crystalline structure is what primarily dictates its solid nature. Boron's atoms are arranged in a complex, icosahedral structure—a three-dimensional geometric shape with 20 faces and 12 vertices. These icosahedra are linked together in various ways, creating a robust and rigid network. This unique structure contributes to boron's high hardness and high melting point. The strong covalent bonds between boron atoms are responsible for the stability of its solid form under normal conditions.
The High Melting Point of Boron: A Consequence of Strong Bonding
Boron possesses an exceptionally high melting point, around 2076 °C (3769 °F). This high melting point is a direct consequence of the strong covalent bonds within its icosahedral structure. It takes a significant amount of energy to overcome these strong bonds and transition from the solid to the liquid phase. This contrasts sharply with many other elements, which have significantly lower melting points due to weaker interatomic forces.
Boron's Boiling Point: The Transition to the Gaseous State
Boron's boiling point is even higher than its melting point, estimated to be around 4000 °C (7232 °F). This extremely high boiling point again emphasizes the strength of the covalent bonds holding its structure together. Reaching this temperature requires an immense amount of energy to completely overcome the interatomic forces and transition boron to a gaseous state. In practical terms, achieving and maintaining such temperatures is incredibly challenging.
Boron under Extreme Conditions: Exploring Possibilities
While boron is predominantly a solid under normal conditions, exploring its behavior under extreme conditions is crucial to gain a more complete understanding of its potential states. High temperatures and pressures could potentially influence its properties and perhaps allow observation of different states of matter.
High-Temperature Behavior: Is Liquid Boron Possible?
While achieving the boiling point of boron is extremely difficult, liquid boron can be obtained at temperatures above its melting point. However, due to its high reactivity at these temperatures, studying liquid boron often involves specialized techniques and controlled environments. The challenges lie not only in reaching and maintaining such extreme temperatures but also in preventing unwanted reactions with the surrounding environment.
High-Pressure Scenarios: Altering Boron's Structure
Applying immense pressure could potentially alter boron's crystalline structure. This could lead to changes in its physical properties, potentially influencing its density, hardness, and other characteristics. Research into high-pressure boron is an ongoing area of scientific investigation, potentially revealing novel forms and properties of this element.
Amorphous Boron: A Different Perspective
It's important to distinguish between crystalline boron and amorphous boron. Crystalline boron refers to the ordered icosahedral structure described above. Amorphous boron, however, lacks this long-range order. Its atoms are arranged randomly, similar to glass. Amorphous boron is less dense and has different physical properties compared to its crystalline counterpart. The production methods heavily influence whether the resultant boron is crystalline or amorphous.
Applications of Boron: Harnessing its Unique Properties
Boron's unique properties make it invaluable in various applications:
- High-strength materials: Its hardness and high melting point make boron crucial in developing high-strength materials for aerospace and military applications.
- Semiconductors: Boron's semiconducting properties are vital in the electronics industry, used in semiconductors and transistors.
- Nuclear reactors: Boron's ability to absorb neutrons makes it useful in controlling nuclear reactions.
- Glass and ceramics: Boron compounds are used in the production of specialized glasses and ceramics with improved properties.
Conclusion: Boron Remains Primarily a Solid
In conclusion, under normal conditions, boron exists unequivocally as a solid. Its complex crystalline structure, high melting point, and high boiling point solidify its status as a solid at standard temperature and pressure. While theoretically it can exist in liquid and gaseous states under extreme conditions, the extreme temperatures and pressures required make these states challenging to achieve and study. Therefore, considering everyday situations and practical applications, boron is firmly classified as a solid. The unique properties stemming from its solid nature are at the core of its widespread applications in various industrial and technological fields.
Understanding boron’s behavior under extreme conditions remains an active area of scientific research, promising to unravel further aspects of this intriguing metalloid's diverse properties. This ongoing research might reveal further nuances about its behavior in states beyond its primary solid form, possibly altering our understanding of boron's potential across various applications and scientific fields. The intricacies of boron’s characteristics are a testament to the complexity and wonder of the elements and their behavior under various conditions.
Latest Posts
Latest Posts
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Boron A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
