Is Boiling Water Chemical Or Physical Change
News Leon
Mar 14, 2025 · 5 min read
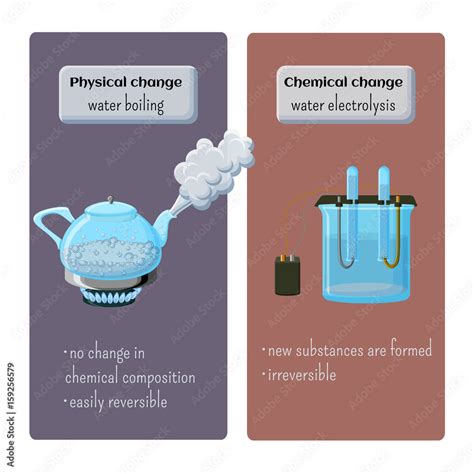
Table of Contents
Is Boiling Water a Chemical or Physical Change? A Comprehensive Exploration
The question of whether boiling water represents a chemical or physical change is a fundamental one in chemistry, often sparking debate among students and enthusiasts alike. While seemingly simple, understanding the distinction requires a deep dive into the concepts of chemical and physical changes, and a closer look at the molecular behavior of water during the boiling process. This article will comprehensively explore this topic, clarifying the nature of boiling water and highlighting the key differences between chemical and physical transformations.
Understanding Chemical vs. Physical Changes
Before delving into the specifics of boiling water, it's crucial to establish a clear understanding of the core differences between chemical and physical changes.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the alteration of the chemical composition of a substance. This means that the atoms within the substance rearrange themselves to form new molecules with different properties. Key indicators of a chemical change include:
- Formation of a new substance: The product(s) have distinctly different properties than the reactants.
- Change in color: A noticeable shift in color often indicates a chemical reaction.
- Release or absorption of heat (energy): Exothermic reactions release heat, while endothermic reactions absorb heat.
- Formation of a gas: The production of bubbles or fumes can signify a chemical reaction.
- Formation of a precipitate: The appearance of a solid from a solution is a hallmark of some chemical reactions.
Physical Changes: Altering Appearance, Not Composition
In contrast, a physical change alters the physical properties of a substance, such as its shape, size, or state (solid, liquid, gas), without altering its chemical composition. The fundamental building blocks (atoms and molecules) remain the same. Examples of physical changes include:
- Melting: Changing from a solid to a liquid (e.g., ice melting).
- Freezing: Changing from a liquid to a solid (e.g., water freezing).
- Boiling/Evaporation: Changing from a liquid to a gas (e.g., water boiling).
- Condensation: Changing from a gas to a liquid (e.g., steam condensing).
- Sublimation: Changing from a solid directly to a gas (e.g., dry ice).
- Dissolving: Mixing a substance into a liquid without changing its chemical composition (e.g., salt dissolving in water).
The Boiling Process: A Detailed Look at Water
Now, let's examine the process of boiling water through the lens of chemical and physical changes. When water boils, it transitions from a liquid state to a gaseous state (steam). This transformation is characterized by several key observations:
- Temperature remains constant: During boiling, the temperature of the water remains at 100°C (212°F) at standard atmospheric pressure, even as heat continues to be added. This is because the added energy is used to overcome the intermolecular forces holding the water molecules together in the liquid phase, allowing them to escape as gas.
- Change in state: The water's physical state changes from liquid to gas, a clear indication of a physical change.
- No new substance is formed: The steam produced is still composed of H₂O molecules; no new chemical bonds are formed or broken. The molecules simply gain enough kinetic energy to overcome the attractive forces keeping them in a liquid state.
- Reversible process: The process of boiling is easily reversible. By cooling the steam, it condenses back into liquid water, demonstrating the preservation of the original chemical composition.
Therefore, boiling water is unequivocally a physical change.
Misconceptions and Clarifications
Despite the clear evidence, some misconceptions may arise concerning the boiling process:
Misconception 1: The presence of bubbles indicates a chemical reaction.
Clarification: Bubbles in boiling water are simply steam (water vapor) forming and rising to the surface. They are not indicative of a new substance forming. The bubbles are composed of water molecules in the gaseous phase, not a chemically different compound.
Misconception 2: The change in volume suggests a chemical reaction.
Clarification: The expansion of volume when water boils is a result of the change in state. Gaseous water molecules require significantly more space than liquid water molecules due to the weaker intermolecular forces. This is a physical property change, not a chemical one.
Misconception 3: Any change involving heat is a chemical change.
Clarification: Many physical changes involve the absorption or release of heat (like melting ice or boiling water). Heat is a form of energy and is involved in many physical processes, not just chemical ones. The key difference lies in whether the chemical composition changes.
Analogies and Real-World Examples
To further solidify the understanding, consider these analogies:
- Ice melting: Similar to boiling, melting ice is a physical change. The water molecules remain H₂O, regardless of whether they are arranged in a solid crystal lattice or moving freely in a liquid state.
- Salt dissolving in water: Dissolving salt in water is another example of a physical change. The salt molecules are separated and surrounded by water molecules, but the chemical composition of both salt and water remains unchanged. Evaporation of the water would leave behind the original salt.
- Sugar dissolving in water: Similar to salt, dissolving sugar in water is a physical change. The sugar molecules are dispersed in the water, but their chemical structure remains intact.
The Importance of Understanding the Difference
Distinguishing between chemical and physical changes is crucial for several reasons:
- Scientific understanding: It forms the foundation for understanding chemical reactions and physical phenomena.
- Industrial processes: Understanding these differences is critical in various industrial processes, from material science to food processing.
- Environmental science: Differentiating between chemical and physical changes plays a key role in assessing environmental impact.
- Everyday life: Many everyday occurrences involve physical and chemical changes, and understanding the difference can improve our interaction with the world around us.
Conclusion: Boiling Water - A Physical Transformation
In conclusion, the process of boiling water is undeniably a physical change. While it involves a change in state, temperature, and volume, it doesn't alter the chemical composition of the water. The water molecules remain H₂O throughout the process. Understanding this distinction highlights the importance of precise definitions in science and reinforces the fundamental principles governing physical and chemical transformations. The process is reversible, easily demonstrating the absence of a fundamental chemical alteration. Boiling water serves as a simple yet powerful illustration of the key differences between physical and chemical changes, forming a cornerstone for understanding more complex chemical and physical phenomena. Remember, the focus is on whether the chemical composition changes – not just the observable physical properties.
Latest Posts
Latest Posts
-
Transactions Are Recorded In A Journal In
Mar 14, 2025
-
Which Of The Following Needs A Proof
Mar 14, 2025
-
Pure Substances Are Made Of Only One Type Of
Mar 14, 2025
-
A Dpt Vaccination Is An Example Of
Mar 14, 2025
-
Whats The Difference Between An Enzyme And A Hormone
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
