Is Boiling Water A Physical Change Or A Chemical Change
News Leon
Mar 14, 2025 · 4 min read
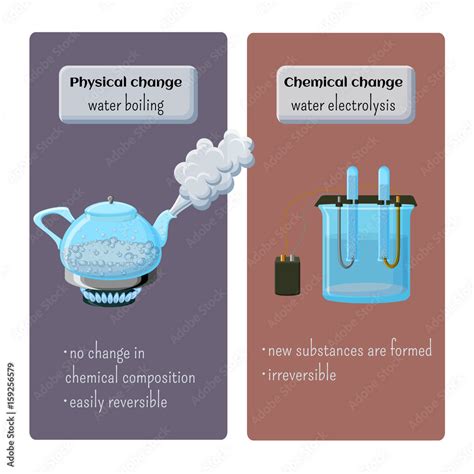
Table of Contents
Is Boiling Water a Physical Change or a Chemical Change? A Deep Dive
The question of whether boiling water represents a physical or chemical change is a fundamental one in science, often encountered early in a student's scientific journey. While seemingly simple, a thorough understanding requires delving into the core concepts of matter, its states, and the processes that transform it. This article aims to comprehensively address this question, exploring the nuances and providing a clear, definitive answer.
Understanding Physical and Chemical Changes
Before diving into the specifics of boiling water, let's establish a clear understanding of the difference between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same, just in a different state or form. Examples include:
- Changes of state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and deposition (gas to solid). These changes involve altering the arrangement of molecules but not their fundamental structure.
- Shape changes: Crushing a can, cutting paper, bending a wire. The material's chemical makeup remains unaltered.
- Dissolution: Dissolving salt in water. The salt particles are dispersed, but the salt itself remains chemically unchanged; it can be recovered through evaporation.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. This transformation occurs due to the breaking and forming of chemical bonds, resulting in a change in the molecular structure. Examples include:
- Combustion: Burning wood or paper. The original materials are transformed into ash, gases (like carbon dioxide and water vapor), and other byproducts.
- Rusting: Iron reacting with oxygen and water to form iron oxide (rust). The iron's chemical composition is altered.
- Cooking an egg: The proteins in the egg undergo irreversible chemical changes due to heat, resulting in a solidified structure.
Analyzing the Boiling Process
Now, let's specifically analyze what happens when water boils. When water is heated, its temperature increases, causing its molecules to gain kinetic energy. This increased kinetic energy overcomes the intermolecular forces holding the water molecules together in the liquid state.
The Molecular Perspective
As the water reaches its boiling point (100°C or 212°F at standard atmospheric pressure), the kinetic energy of the molecules becomes sufficient to break free from the liquid phase and transition into the gaseous phase (steam). This phase transition is characterized by a significant increase in the distance between water molecules. However, the chemical composition of the water molecules remains unchanged. Each water molecule (H₂O) still consists of two hydrogen atoms covalently bonded to one oxygen atom. There's no alteration in the molecular structure; no new chemical bonds are formed, and no existing ones are broken.
Observable Changes vs. Chemical Composition
While boiling water results in observable changes – liquid water transforming into steam – these changes are purely physical. We see a change in state, a change in volume, and a change in density. However, the chemical identity of the substance remains consistent throughout the process. If you were to condense the steam back into liquid water, you would have the same water you started with. This reversibility is a key indicator of a physical change.
Debunking Misconceptions
It's important to address some common misconceptions that might lead to the incorrect classification of boiling water as a chemical change.
The Production of Steam
Some might argue that the formation of steam (water vapor) signifies a new substance, thus implying a chemical change. However, steam is merely water in a gaseous state. It possesses the same chemical composition as liquid water (H₂O). The change is a physical transformation, not a chemical transformation.
Energy Input
The fact that energy (heat) is required to boil water might lead some to believe that a chemical reaction is occurring. However, energy input is frequently involved in physical changes. For example, melting ice also requires energy input. The energy is used to overcome intermolecular forces, not to break chemical bonds.
Supporting Evidence for Physical Change
Several lines of evidence strongly support the classification of boiling water as a physical change:
- Reversibility: The process is reversible. By cooling the steam, we can easily condense it back into liquid water, regaining the original substance. This reversibility is a hallmark of physical changes.
- Chemical properties remain unchanged: The water molecules retain their inherent properties, such as their polar nature and their ability to dissolve certain substances. If a chemical change had occurred, these properties would likely be altered.
- No new substances are formed: Only the physical state of the water changes; no new compounds or elements are produced during the boiling process.
Conclusion: Boiling Water is a Physical Change
In conclusion, the process of boiling water is unequivocally a physical change. While the state of water changes from liquid to gas, its chemical composition remains unchanged. The transformation involves a change in the arrangement and kinetic energy of water molecules, but not a change in their fundamental chemical structure. Understanding this distinction is crucial for grasping fundamental concepts in chemistry and physics. It highlights the importance of distinguishing between observable changes and alterations in the underlying chemical composition of a substance.
Latest Posts
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Physical Change Or A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.