Is Boiling Water A Chemical Change Or Physical Change
News Leon
Mar 14, 2025 · 5 min read
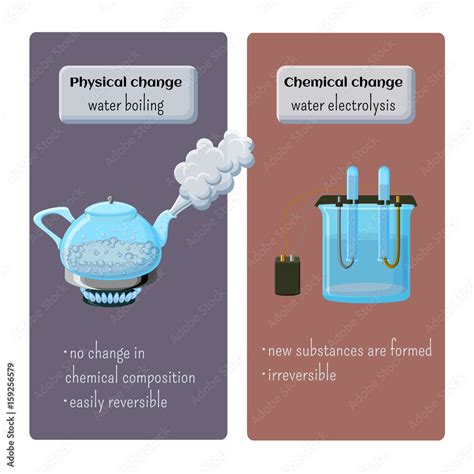
Table of Contents
Is Boiling Water a Chemical Change or a Physical Change? A Comprehensive Look
The question of whether boiling water represents a chemical or physical change is a fundamental one in understanding the nature of matter and its transformations. While seemingly simple, a thorough examination reveals a deeper understanding of the processes involved and the subtle distinctions between physical and chemical changes. This article will delve into this question, exploring the definitions, the evidence, and the implications of classifying boiling water.
Understanding Chemical vs. Physical Changes
Before we analyze the boiling of water, let's establish clear definitions of chemical and physical changes. This is crucial for accurate classification.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new substances with different chemical properties. Key indicators of a chemical change include:
- Formation of a new substance: The product(s) have different properties than the reactants.
- Change in color: A noticeable alteration in hue often suggests a chemical transformation.
- Production of gas: The release of bubbles or fumes points towards a chemical reaction.
- Formation of a precipitate: The formation of a solid from a solution.
- Release or absorption of heat: Exothermic (heat released) and endothermic (heat absorbed) reactions are common in chemical changes.
- Irreversibility (often): Many chemical changes are difficult or impossible to reverse easily.
Physical Changes: Altering Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules remain the same, though their arrangement or state may alter. Examples of physical changes include:
- Change of state: Melting, freezing, boiling, condensation, sublimation, and deposition.
- Dissolving: A substance dissolving in a solvent, forming a solution.
- Cutting or crushing: Changes in shape or size without altering the chemical makeup.
- Changes in density or volume: These are often reversible.
Analyzing the Boiling of Water
Now, let's apply these definitions to the boiling of water. When water boils, it transitions from its liquid state to its gaseous state (steam). This is a change of state, a classic example often cited in discussions of physical changes.
Evidence Supporting a Physical Change
Several pieces of evidence strongly support the classification of boiling water as a physical change:
- No new substance is formed: Steam (water vapor) is still H₂O. The chemical formula remains unchanged. The molecules are simply further apart and moving at higher speeds.
- Reversible process: Condensation, the reverse of boiling, readily converts steam back into liquid water. This reversibility is a hallmark of physical changes.
- No significant color change: Liquid water and steam are both colorless.
- No noticeable gas production (other than water vapor): While steam is a gas, it's just the water itself in a different state. There's no release of other gases.
- Heat absorption: Boiling is an endothermic process; it absorbs heat to transition to the gaseous phase. While heat is involved, this is a characteristic of many physical changes.
Addressing Potential Misconceptions
Some might argue that the energy input during boiling could initiate a chemical change. However, the energy primarily overcomes the intermolecular forces holding the water molecules together in the liquid phase. It doesn't break the covalent bonds within the H₂O molecule itself. The O-H bonds remain intact.
Dissociation of Water: A Complication?
A more nuanced point concerns the slight dissociation of water into hydronium (H₃O⁺) and hydroxide (OH⁻) ions. This is an equilibrium reaction:
2H₂O ⇌ H₃O⁺ + OH⁻
This process occurs to a very small extent in pure water, even at room temperature. Boiling water increases the rate of this dissociation, but the proportion of dissociated water molecules remains extremely low. The overwhelming majority of water molecules remain as H₂O.
Therefore, while a small amount of dissociation occurs, it does not significantly alter the overall classification of boiling as a physical change. The primary transformation is the phase transition, which is fundamentally physical.
The Importance of Context and Precision
While classifying boiling water as a physical change is accurate in most contexts, it’s crucial to acknowledge the subtle nuances. The slight dissociation of water is a chemical process, but its contribution to the overall change is negligible compared to the dominant physical process of vaporization. Scientific language necessitates precision, and acknowledging the minor chemical aspect allows for a more complete description.
For most practical purposes, including educational contexts at introductory levels, classifying boiling water as a physical change is perfectly acceptable and accurate. The emphasis should be on the dominant process, the phase transition, which unequivocally constitutes a physical change.
Beyond Boiling: Other Phase Transitions
The principles discussed regarding boiling apply to other phase transitions:
- Melting (solid to liquid): Ice melting into water is a physical change.
- Freezing (liquid to solid): Water freezing into ice is a physical change.
- Sublimation (solid to gas): Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas is a physical change.
- Deposition (gas to solid): Frost forming on a cold surface is a physical change.
In all these instances, the chemical composition of the substance remains unchanged; only its physical state alters.
Conclusion: Boiling Water – Primarily a Physical Change
In conclusion, the boiling of water is primarily a physical change. While a minor degree of chemical dissociation occurs, this is overshadowed by the major transformation—the phase transition from liquid to gas—which is unequivocally a physical process. The molecules remain the same; only their arrangement and state change. Understanding this distinction is key to grasping the fundamental concepts of matter and its transformations. The precision of scientific terminology requires acknowledging the subtle chemical aspect, but for practical purposes and introductory learning, characterizing boiling water as a physical change is entirely appropriate and accurate.
Latest Posts
Latest Posts
-
The Rungs Of The Dna Ladder Are Made Of What
Mar 14, 2025
-
What Is 3 Days In Hours
Mar 14, 2025
-
Which Of The Following Is Not A Renewable Energy Source
Mar 14, 2025
-
How Many Inches Are In One Cubic Foot
Mar 14, 2025
-
How Many Diodes Are Required To Form A Bridge Rectifier
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Chemical Change Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
