Is Baking Soda An Element Or Compound
News Leon
Mar 14, 2025 · 6 min read
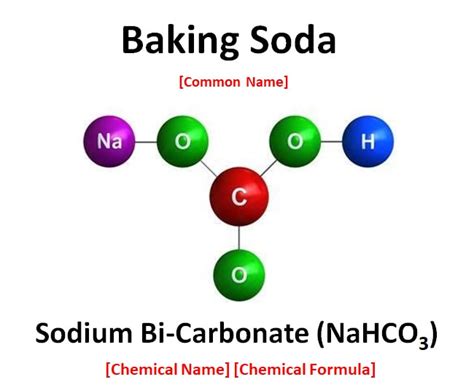
Table of Contents
- Is Baking Soda An Element Or Compound
- Table of Contents
- Is Baking Soda an Element or Compound? A Deep Dive into Chemical Composition
- Understanding Elements and Compounds
- Elements: The Building Blocks of Matter
- Compounds: A Combination of Elements
- Baking Soda: A Chemical Compound
- The Properties of Sodium Bicarbonate (Baking Soda)
- Physical Properties:
- Chemical Properties:
- Baking Soda's Chemical Reactions: A Closer Look
- Reaction with Acids: The Leavening Process
- Thermal Decomposition: A Secondary Leavening Mechanism
- Baking Soda's Diverse Applications
- In Baking and Cooking:
- In Cleaning and Personal Care:
- In Other Applications:
- Distinguishing Baking Soda from Baking Powder
- Conclusion: Baking Soda – A Remarkable Compound
- Latest Posts
- Latest Posts
- Related Post
Is Baking Soda an Element or Compound? A Deep Dive into Chemical Composition
Baking soda, a ubiquitous ingredient in kitchens worldwide, sparks curiosity beyond its leavening power. Many wonder: is baking soda an element or a compound? The answer, as we'll explore in detail, is far more nuanced than a simple yes or no. Understanding this requires delving into the fundamental concepts of chemistry, specifically the definitions of elements and compounds. This article will not only answer the central question but also explore the properties of baking soda, its chemical reactions, and its widespread applications.
Understanding Elements and Compounds
Before we classify baking soda, let's establish clear definitions:
Elements: The Building Blocks of Matter
An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. This number, called the atomic number, uniquely identifies each element. Elements are the fundamental building blocks of all matter; they cannot be broken down into simpler substances by chemical means. Examples include hydrogen (H), oxygen (O), and iron (Fe). The periodic table organizes and displays all known elements.
Compounds: A Combination of Elements
A compound, in contrast, is a substance formed when two or more chemical elements are chemically bonded together. These bonds create a new substance with unique properties different from its constituent elements. Compounds can be broken down into their constituent elements through chemical reactions, but not through physical processes like filtration or distillation. Water (H₂O), for instance, is a compound composed of hydrogen and oxygen atoms chemically bonded together. The properties of water are vastly different from those of hydrogen gas and oxygen gas.
Baking Soda: A Chemical Compound
Now, armed with these definitions, we can definitively state that baking soda is a compound, not an element. Its chemical name is sodium bicarbonate, and its chemical formula is NaHCO₃. This formula reveals that baking soda is composed of three different elements:
- Sodium (Na): An alkali metal, highly reactive with water.
- Hydrogen (H): The lightest element, a key component of many organic molecules.
- Carbon (C): A nonmetal crucial for life and found in organic compounds.
- Oxygen (O): A highly reactive nonmetal, essential for respiration and numerous chemical processes.
These elements are chemically bonded together in a specific ratio, forming a distinct crystalline structure with unique physical and chemical properties. It's impossible to simply separate sodium, hydrogen, carbon, and oxygen from baking soda through physical means. Chemical reactions are required to break the bonds and isolate the constituent elements.
The Properties of Sodium Bicarbonate (Baking Soda)
Baking soda's usefulness stems from its unique properties, many of which are a direct consequence of its chemical composition:
Physical Properties:
- Appearance: White, fine crystalline powder.
- Odor: Odorless.
- Taste: Slightly salty and alkaline.
- Solubility: Soluble in water, slightly soluble in alcohol.
- Melting Point: Decomposes before melting at approximately 50°C (122°F).
Chemical Properties:
- Alkalinity: Baking soda is a weak base, meaning it can neutralize acids. This is the basis for its use as a leavening agent in baking. When combined with an acid (like vinegar or buttermilk), it undergoes a chemical reaction that produces carbon dioxide gas, causing dough or batter to rise.
- Reactivity with Acids: As mentioned, baking soda reacts with acids to produce carbon dioxide, water, and a salt. This reaction is exothermic, meaning it releases heat.
- Thermal Decomposition: When heated to high temperatures, baking soda decomposes into sodium carbonate (washing soda), carbon dioxide, and water. This is another reason it's effective as a leavening agent.
- Mild Abrasiveness: Its slightly abrasive nature makes it useful as a cleaning agent.
Baking Soda's Chemical Reactions: A Closer Look
Let's delve deeper into the key chemical reactions involving baking soda:
Reaction with Acids: The Leavening Process
The most significant reaction of baking soda is its interaction with acids. This reaction is fundamental to its use as a leavening agent in baking. The general reaction can be represented as:
NaHCO₃ (baking soda) + HA (acid) → NaA (salt) + H₂O (water) + CO₂ (carbon dioxide)
Where HA represents a generic acid, and NaA is the resulting salt.
For example, the reaction with vinegar (acetic acid) is:
NaHCO₃ + CH₃COOH → CH₃COONa + H₂O + CO₂
This reaction produces carbon dioxide gas, which gets trapped within the batter or dough, causing it to rise and become light and fluffy. The rate of this reaction can be controlled by the type and concentration of acid used, as well as the temperature.
Thermal Decomposition: A Secondary Leavening Mechanism
Baking soda also undergoes thermal decomposition when heated:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂
This reaction produces sodium carbonate (washing soda), water, and carbon dioxide. The carbon dioxide released contributes to leavening, especially in recipes where the reaction with an acid is limited or slow. However, this decomposition can also impart a slightly bitter taste if too much sodium carbonate is formed.
Baking Soda's Diverse Applications
The unique properties of baking soda extend its uses far beyond the kitchen:
In Baking and Cooking:
- Leavening agent: Creates light and airy textures in baked goods like cakes, cookies, and bread.
- Neutralizing agent: Balances acidity in certain recipes, preventing bitterness.
- Tenderizer: Helps tenderize meats by partially breaking down protein molecules.
In Cleaning and Personal Care:
- Deodorizer: Neutralizes odors in refrigerators, carpets, and other spaces.
- Cleaning agent: Effectively removes stains and grease from surfaces.
- Abrasive cleanser: Gently scrubs away grime and stains without scratching.
- Toothpaste ingredient: Helps whiten teeth and neutralize acids.
In Other Applications:
- Fire extinguisher: Used in some fire extinguishers due to its ability to release carbon dioxide.
- Antacid: Neutralizes stomach acid, providing relief from heartburn and indigestion.
- Industrial uses: Employed in various industrial processes, including the manufacturing of plastics and pharmaceuticals.
Distinguishing Baking Soda from Baking Powder
It's crucial to distinguish between baking soda and baking powder. While both are leavening agents, they differ significantly in their composition and use:
- Baking soda is pure sodium bicarbonate. It requires an acid to activate and produce carbon dioxide.
- Baking powder contains baking soda and an acid (usually cream of tartar) along with a drying agent (like cornstarch). It's a complete leavening system; no additional acid is needed.
Conclusion: Baking Soda – A Remarkable Compound
In conclusion, baking soda is unequivocally a compound, not an element. Its chemical formula, NaHCO₃, clearly demonstrates its composition of four different elements – sodium, hydrogen, carbon, and oxygen – chemically bonded together. Its unique properties, stemming from this composition, render it a versatile substance with widespread applications in baking, cleaning, personal care, and various industrial processes. Understanding its chemical nature and reactions is key to appreciating its multifaceted utility and harnessing its potential effectively. The versatility of this seemingly simple compound underscores the fascinating complexities of chemistry and the profound impact of chemical reactions on our daily lives. From fluffy cakes to sparkling clean surfaces, baking soda’s role is undeniable, highlighting its importance as a cornerstone of both culinary practices and household utility.
Latest Posts
Latest Posts
-
The Amount Of Space Occupied By A Substance Is Its
Mar 15, 2025
-
Any Computer Parts You Can Touch
Mar 15, 2025
-
The Law Of Diminishing Marginal Utility States That
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda An Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
