Is A Hydrogen Bond Stronger Than A Covalent Bond
News Leon
Mar 22, 2025 · 5 min read
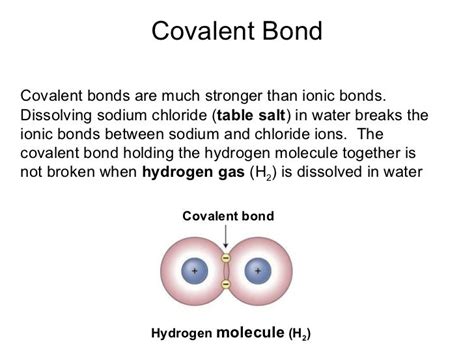
Table of Contents
Is a Hydrogen Bond Stronger Than a Covalent Bond? A Deep Dive into Chemical Bonds
The question of whether a hydrogen bond is stronger than a covalent bond is a fundamental one in chemistry, often misunderstood due to the subtle differences in their nature and strength. The simple answer is no, a hydrogen bond is significantly weaker than a covalent bond. However, understanding why requires a deeper exploration of the characteristics of each bond type. This article delves into the intricacies of covalent and hydrogen bonds, comparing their strengths, properties, and the crucial role they play in various chemical and biological systems.
Understanding Covalent Bonds: The Foundation of Molecular Structure
Covalent bonds are the bedrock of molecular structure, formed when two atoms share one or more pairs of electrons. This sharing creates a strong attractive force between the atoms, holding them together to form molecules. The strength of a covalent bond depends on several factors:
-
Electronegativity: The tendency of an atom to attract electrons towards itself. A larger difference in electronegativity between the atoms involved leads to a more polar covalent bond, while a smaller difference results in a nonpolar covalent bond. However, even polar covalent bonds are still fundamentally stronger than hydrogen bonds.
-
Bond Order: The number of electron pairs shared between two atoms. A higher bond order (e.g., double or triple bonds) indicates a stronger bond. Think of a double bond as two single bonds working in tandem – double the strength!
-
Atomic Size: Smaller atoms generally form stronger covalent bonds because the shared electrons are closer to the nuclei, experiencing a stronger electrostatic attraction.
Examples of Covalent Bonds: The bonds in water (H₂O), methane (CH₄), and oxygen gas (O₂) are all prime examples of covalent bonds. The strong covalent bonds within these molecules give them distinct properties and stability.
Covalent Bond Strength: Measured in Energy
The strength of a covalent bond is typically quantified in terms of bond dissociation energy, the amount of energy required to break a single bond and separate the atoms. This energy is usually expressed in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Bond dissociation energies for typical covalent bonds range from several hundred kJ/mol to over a thousand kJ/mol, highlighting their robust nature.
Hydrogen Bonds: A Special Type of Intermolecular Force
Unlike covalent bonds, which involve the sharing of electrons within a molecule, hydrogen bonds are a type of intermolecular force, meaning they occur between molecules. They arise when a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This attraction is due to the partial positive charge (δ+) on the hydrogen atom and the partial negative charge (δ-) on the electronegative atom.
Characteristics of Hydrogen Bonds:
-
Weaker than Covalent Bonds: This is the key difference. Hydrogen bonds are significantly weaker than covalent bonds, typically having bond energies in the range of 10-40 kJ/mol.
-
Directional: The bond strength is maximized when the hydrogen atom and the two electronegative atoms are in a straight line.
-
Important Biological Roles: Hydrogen bonds play a vital role in many biological systems, including:
- DNA Structure: Hydrogen bonds hold together the two strands of the DNA double helix.
- Protein Folding: Hydrogen bonds contribute to the specific three-dimensional structure of proteins, essential for their function.
- Water Properties: Hydrogen bonds are responsible for many of water's unique properties, such as its high boiling point and surface tension.
Understanding the Hydrogen Bond's Weakness:
The relative weakness of the hydrogen bond stems from the fact that it's an electrostatic interaction between partially charged atoms, rather than the direct sharing of electrons as in a covalent bond. While the electrostatic attraction is considerable, it's not as strong as the powerful force resulting from the electron sharing in covalent bonding.
Quantitative Comparison: Covalent vs. Hydrogen Bond Strengths
Let's compare some typical bond energies to solidify the concept:
| Bond Type | Bond Example | Approximate Bond Energy (kJ/mol) |
|---|---|---|
| Covalent | O-H (in water) | 463 |
| Covalent | C-H (in methane) | 413 |
| Covalent | C=O (in ketones) | 745 |
| Hydrogen Bond | O-H···O (water) | 20-30 |
The table clearly demonstrates that covalent bonds have considerably higher bond energies than hydrogen bonds. The energy required to break a covalent bond is far greater than that needed to break a hydrogen bond.
The Importance of Hydrogen Bonds Despite Their Weakness
Despite their relative weakness, hydrogen bonds are crucial for life and many chemical processes. Their weaker nature allows for dynamic interactions. For example, the ability of DNA strands to separate and rejoin easily is directly related to the relatively weak nature of the hydrogen bonds connecting them. This dynamic behavior is essential for DNA replication and transcription. Similarly, the ease with which hydrogen bonds can form and break enables proteins to fold and unfold, crucial for their function and regulation.
The collective strength of many hydrogen bonds can be substantial. While individual hydrogen bonds are weak, a vast network of them working together can contribute significantly to the overall stability of a system. This is particularly evident in the three-dimensional structures of proteins and nucleic acids.
Conclusion: A Matter of Perspective
While a single hydrogen bond is significantly weaker than a single covalent bond, their roles in chemistry and biology are fundamentally different and equally important. Covalent bonds form the strong backbone of molecules, defining their structure and stability. Hydrogen bonds, on the other hand, are responsible for essential intermolecular interactions, shaping larger-scale structures and influencing dynamic processes critical to life. Therefore, the comparison should not be seen as a simple matter of "stronger" or "weaker," but rather a recognition of their distinct roles and contributions to the chemical world. Understanding this difference is key to appreciating the complexity and beauty of chemical bonding.
Latest Posts
Latest Posts
-
In Glycolysis There Is A Net Gain Of Atp
Mar 22, 2025
-
Which Of The Following Is Not True Of Rna Processing
Mar 22, 2025
-
Is Burning Coal A Chemical Change
Mar 22, 2025
-
An Alpha Particle Travels At A Velocity Of Magnitude 550
Mar 22, 2025
-
Which Of The Following Is An Experiment
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is A Hydrogen Bond Stronger Than A Covalent Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
