In An Oxidation Reduction Reaction The Substance Oxidized Always
News Leon
Apr 06, 2025 · 6 min read
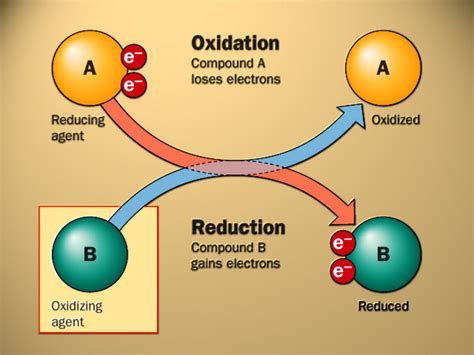
Table of Contents
In an Oxidation-Reduction Reaction, the Substance Oxidized Always… Loses Electrons!
Oxidation-reduction reactions, also known as redox reactions, are fundamental processes in chemistry and biology. Understanding these reactions is crucial for comprehending a vast array of phenomena, from combustion and corrosion to respiration and photosynthesis. A core concept within redox reactions is the principle that in an oxidation-reduction reaction, the substance oxidized always loses electrons. This seemingly simple statement underpins the entire framework of redox chemistry. Let's delve deeper into this concept and explore its implications.
Understanding Oxidation and Reduction
Before we solidify the understanding that the substance oxidized always loses electrons, let's first clearly define oxidation and reduction. These terms are often confusing for beginners, but a simple mnemonic device can help: OIL RIG.
- OIL: Oxidation Is Loss (of electrons)
- RIG: Reduction Is Gain (of electrons)
Oxidation involves the loss of electrons by an atom, ion, or molecule. This loss results in an increase in the oxidation state (oxidation number) of the species involved. Conversely, reduction involves the gain of electrons, leading to a decrease in the oxidation state.
It's crucial to remember that oxidation and reduction always occur simultaneously. You cannot have one without the other. This is because electrons cannot simply disappear or appear out of thin air; they must be transferred from one species to another. Therefore, in every redox reaction, there is a reducing agent (the substance that loses electrons and is itself oxidized) and an oxidizing agent (the substance that gains electrons and is itself reduced).
The Substance Oxidized Always Loses Electrons: A Deeper Dive
The statement "in an oxidation-reduction reaction, the substance oxidized always loses electrons" is a fundamental truth of redox chemistry. It's not just a rule; it's the very definition of oxidation. Let's illustrate this with a few examples.
Example 1: The Reaction Between Zinc and Copper(II) Sulfate
Consider the reaction between zinc metal (Zn) and copper(II) sulfate (CuSO₄) in solution. The balanced equation is:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
In this reaction:
- Zinc (Zn) loses two electrons to become a Zn²⁺ ion: Zn → Zn²⁺ + 2e⁻. This is oxidation – zinc is the reducing agent.
- Copper(II) ions (Cu²⁺) gain two electrons to become copper metal (Cu): Cu²⁺ + 2e⁻ → Cu. This is reduction – copper(II) ions are the oxidizing agent.
Notice that the electrons lost by zinc are precisely the electrons gained by copper(II) ions. The number of electrons lost must always equal the number of electrons gained to maintain charge balance. This directly exemplifies the principle that the substance oxidized (zinc) always loses electrons.
Example 2: Combustion of Methane
The combustion of methane (CH₄) is another classic example of a redox reaction:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
In this reaction:
- Carbon in methane (CH₄) has an oxidation state of -4. In carbon dioxide (CO₂), its oxidation state is +4. Therefore, carbon has undergone oxidation (loss of electrons).
- Oxygen in O₂ has an oxidation state of 0. In both CO₂ and H₂O, oxygen's oxidation state is -2. Therefore, oxygen has undergone reduction (gain of electrons).
Again, we see the clear connection: the substance oxidized (carbon) loses electrons, while the substance reduced (oxygen) gains them. The total number of electrons transferred is balanced throughout the reaction.
Example 3: The Rusting of Iron
The rusting of iron (Fe) is a common example of redox reaction, driven by the oxidation of iron by oxygen in the presence of water:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
Here, iron (Fe) loses electrons to form iron(III) hydroxide (Fe(OH)₃), demonstrating oxidation. Oxygen gains electrons to form water and hydroxide ions, demonstrating reduction. The substance oxidized (iron) always loses electrons in this process.
Identifying Oxidation and Reduction in Complex Reactions
While the examples above are relatively straightforward, identifying oxidation and reduction in more complex reactions can be challenging. However, the fundamental principle remains the same: the substance oxidized always loses electrons. Here's how to approach more complex scenarios:
- Assign oxidation states: Assign oxidation numbers to all atoms in the reactants and products. This allows you to track the changes in oxidation state that occur during the reaction.
- Identify changes in oxidation state: Determine which atoms have increased their oxidation state (oxidation) and which atoms have decreased their oxidation state (reduction).
- Confirm electron transfer: Ensure that the number of electrons lost during oxidation equals the number of electrons gained during reduction. This confirms the balancing of electron transfer within the redox reaction.
Practical Applications of Redox Reactions
The principle that the substance oxidized always loses electrons is not just a theoretical concept; it has widespread practical applications across various fields:
- Batteries: Batteries rely on redox reactions to generate electricity. The oxidation and reduction half-reactions occur at different electrodes, resulting in a flow of electrons.
- Corrosion: Corrosion is a redox reaction where a metal loses electrons to oxygen, leading to the formation of metal oxides. Understanding this process is crucial for developing corrosion-resistant materials.
- Respiration: Cellular respiration is a complex series of redox reactions that produce energy in living organisms. Glucose is oxidized, and oxygen is reduced.
- Photosynthesis: Photosynthesis is also a redox process, where water is oxidized, and carbon dioxide is reduced to form glucose.
- Industrial processes: Many industrial processes, such as the extraction of metals from their ores, rely on redox reactions.
- Analytical Chemistry: Redox titrations are used to quantitatively determine the concentration of various substances.
Beyond the Basics: Half-Reactions and Balancing Redox Equations
Understanding half-reactions is vital for a complete grasp of redox reactions. A half-reaction shows either the oxidation or reduction process in isolation. For instance, in the zinc-copper reaction, the half-reactions are:
- Oxidation: Zn → Zn²⁺ + 2e⁻
- Reduction: Cu²⁺ + 2e⁻ → Cu
Balancing redox equations often requires the use of half-reactions. By balancing the number of electrons lost and gained in the half-reactions, you can then combine them to obtain the balanced overall redox equation. This ensures that the principle of electron conservation is maintained throughout the process, always keeping in mind the central tenet that the substance oxidized always loses electrons.
Conclusion: The Enduring Importance of Electron Transfer
In conclusion, the statement "in an oxidation-reduction reaction, the substance oxidized always loses electrons" is a cornerstone of redox chemistry. It’s not merely a rule to be memorized; it’s a fundamental truth reflecting the nature of electron transfer in chemical reactions. Understanding this principle is essential for comprehending a wide range of chemical and biological processes, from everyday phenomena like rusting to complex industrial applications and life itself. Mastering this concept provides a solid foundation for further exploration of the fascinating world of redox reactions and their significant impact on our lives. Through the consistent application of this foundational principle, we can better analyze, predict, and utilize redox reactions in numerous fields of study and industry. The ability to accurately identify oxidation and reduction based on electron transfer is not just an academic exercise; it is a powerful tool for understanding and manipulating the chemical world around us.
Latest Posts
Latest Posts
-
The Quotient Of 3 And A Number
Apr 06, 2025
-
Where Is The Rhythmicity Center For Respiration
Apr 06, 2025
-
Which Two Elements Most Likely Have The Most Similar Properties
Apr 06, 2025
-
Why Doesnt Hydrogen Have A Neutron
Apr 06, 2025
-
A Number Is Divisible By 2 If
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about In An Oxidation Reduction Reaction The Substance Oxidized Always . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
