Identify The Relationship Between The Following Compounds
News Leon
Mar 22, 2025 · 6 min read
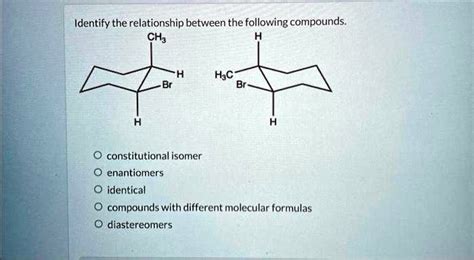
Table of Contents
Identifying Relationships Between Chemical Compounds: A Comprehensive Guide
Understanding the relationships between different chemical compounds is crucial in chemistry. This involves recognizing similarities and differences in their structures, properties, and reactivity. This article delves into various ways to identify these relationships, covering functional groups, isomerism, homologous series, and reaction pathways. We'll explore these concepts with numerous examples, enabling you to confidently analyze and classify chemical compounds.
1. Functional Groups: The Building Blocks of Reactivity
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of that molecule. Identifying the functional group present in a compound is the first step in understanding its behavior and relationship to other compounds. Compounds with the same functional group often exhibit similar chemical properties and undergo similar reactions.
Examples of Functional Groups and their Relationships:
-
Alcohols (-OH): Methanol (CH₃OH), ethanol (CH₃CH₂OH), and propanol (CH₃CH₂CH₂OH) all belong to the alcohol family due to the presence of the hydroxyl (-OH) group. They share similar properties like hydrogen bonding and undergo similar reactions like oxidation.
-
Carboxylic Acids (-COOH): Acetic acid (CH₃COOH), propionic acid (CH₃CH₂COOH), and benzoic acid (C₆H₅COOH) all contain the carboxyl (-COOH) group. They share acidic properties and can undergo esterification reactions.
-
Ketones (C=O): Acetone (CH₃COCH₃), butanone (CH₃CH₂COCH₃), and cyclohexanone (C₆H₁₀O) all have a carbonyl group (C=O) within the carbon chain, making them ketones. They share similar reactivity towards nucleophiles.
-
Alkenes (C=C): Ethene (CH₂=CH₂), propene (CH₃CH=CH₂), and butene (CH₃CH₂CH=CH₂) all contain a carbon-carbon double bond (C=C), characterizing them as alkenes. They undergo addition reactions.
Identifying Relationships based on Functional Groups:
By identifying the functional group, we can predict the likely properties and reactivity of a compound and establish relationships with other compounds sharing the same functional group. For example, knowing that a compound contains an alcohol group suggests it will be polar, capable of hydrogen bonding, and likely to undergo oxidation reactions.
2. Isomerism: Compounds with the Same Formula, Different Structures
Isomers are molecules that have the same molecular formula but different structural arrangements. Different types of isomerism lead to variations in physical and chemical properties.
Types of Isomerism:
-
Structural Isomerism: This involves differences in the arrangement of atoms within the molecule. There are three main types:
- Chain Isomerism: Differing carbon chain structures (e.g., butane and methylpropane).
- Positional Isomerism: Differing positions of a functional group or substituent (e.g., 1-propanol and 2-propanol).
- Functional Group Isomerism: Differing functional groups (e.g., ethanol and dimethyl ether).
-
Stereoisomerism: This involves the same connectivity of atoms but differing spatial arrangements. Key types include:
- Geometric Isomerism (cis-trans isomerism): Differing arrangements of groups around a double bond or ring (e.g., cis- and trans-but-2-ene).
- Optical Isomerism (enantiomerism): Differing spatial arrangements that are non-superimposable mirror images (e.g., L- and D-lactic acid).
Identifying Relationships based on Isomerism:
Isomers provide insights into the effects of structural variations on properties. For example, comparing the boiling points of chain isomers reveals the impact of branching on intermolecular forces. Similarly, comparing the reactivity of geometric isomers shows how the spatial arrangement of groups influences reaction pathways.
3. Homologous Series: Families of Compounds with Similar Structures
A homologous series is a sequence of compounds with the same functional group and similar chemical properties, where each member differs from the next by a CH₂ unit. This consistent structural difference leads to a predictable pattern in physical properties.
Examples of Homologous Series:
-
Alkanes (CnH2n+2): Methane (CH₄), ethane (C₂H₆), propane (C₃H₈), etc. Each member differs by a CH₂ unit, and their boiling points increase gradually with increasing chain length.
-
Alkenes (CnH2n): Ethene (C₂H₄), propene (C₃H₆), butene (C₄H₈), etc. They share the C=C double bond and exhibit similar reactivity.
-
Alcohols (CnH2n+1OH): Methanol (CH₃OH), ethanol (C₂H₅OH), propanol (C₃H₇OH), etc. They share the hydroxyl (-OH) group and show similar properties.
Identifying Relationships based on Homologous Series:
Members of a homologous series exhibit a clear relationship due to their structural similarity and predictable changes in properties with increasing chain length. This allows for the prediction of properties for yet-unsynthesized members of the series.
4. Reaction Pathways: Tracing Relationships Through Chemical Transformations
Chemical reactions provide another avenue for understanding relationships between compounds. A compound's reactivity and the products it forms in specific reactions reveal its relationships with other compounds.
Examples of Reaction Pathways:
-
Oxidation of Alcohols: Primary alcohols oxidize to aldehydes, then to carboxylic acids. Secondary alcohols oxidize to ketones. This series of reactions shows the relationship between alcohols, aldehydes, ketones, and carboxylic acids.
-
Esterification: Carboxylic acids react with alcohols to form esters. This highlights the relationship between carboxylic acids, alcohols, and esters.
-
Addition Reactions of Alkenes: Alkenes undergo addition reactions with various reagents (e.g., hydrogen, halogens, water), forming different products. This shows how alkenes are related to alkanes and other substituted alkanes.
Identifying Relationships based on Reaction Pathways:
By examining the reactions a compound undergoes, we can establish its relationships with reactants and products. This allows us to map out reaction schemes and understand the transformations leading from one compound to another.
5. Spectroscopic Techniques: Unveiling Molecular Structures and Relationships
Spectroscopic techniques, such as Infrared (IR), Nuclear Magnetic Resonance (NMR), and Mass Spectrometry (MS), provide valuable information about the structure of molecules. Comparing spectroscopic data of different compounds allows us to identify similarities and differences in their structures, revealing relationships between them.
Examples of Spectroscopic Analysis:
-
IR Spectroscopy: Identifies functional groups based on characteristic absorption bands. Comparing IR spectra of different alcohols will reveal the presence of the O-H stretching vibration.
-
NMR Spectroscopy: Provides information about the connectivity and environment of atoms in a molecule. Comparing NMR spectra of isomers will show differences in chemical shifts and coupling patterns.
-
Mass Spectrometry: Determines the molecular weight and fragmentation pattern of a molecule. Comparing mass spectra can help identify related compounds.
Identifying Relationships based on Spectroscopic Data:
Spectroscopic data offers definitive structural information, enabling precise comparisons between compounds. Similar spectral features suggest similar structural motifs and thus relationships between compounds.
Conclusion: A Multifaceted Approach to Identifying Relationships
Identifying relationships between chemical compounds requires a multifaceted approach utilizing various tools and techniques. Analyzing functional groups, exploring isomerism, examining homologous series, tracing reaction pathways, and employing spectroscopic techniques provides a comprehensive understanding of the connections between different chemical entities. By integrating these methods, chemists can systematically classify, predict properties, and design synthetic routes for a vast array of chemical compounds. This holistic approach is fundamental to advancements in all areas of chemistry, from organic synthesis to drug discovery and materials science.
Latest Posts
Latest Posts
-
How Many Germ Layers Do Sponges Have
Mar 24, 2025
-
3 Is What Percentage Of 18
Mar 24, 2025
-
A Voltmeter Has An Internal Resistance Of 1000 Ohm
Mar 24, 2025
-
How Many Trips Around The Sun
Mar 24, 2025
-
Python Check If A List Is A Subset Of Another
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Identify The Relationship Between The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
