How To Separate Gasoline And Water
News Leon
Mar 18, 2025 · 6 min read
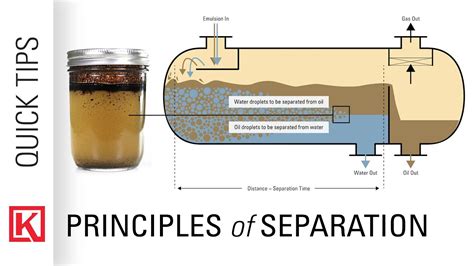
Table of Contents
How to Separate Gasoline and Water: A Comprehensive Guide
Gasoline and water are completely immiscible liquids; this means they don't mix. This property makes separating them relatively straightforward, though the best method depends on the amount of mixture you have and the level of purity required. This comprehensive guide will explore various techniques, from simple household methods ideal for small spills to more sophisticated approaches suitable for larger quantities. We'll also cover safety precautions and considerations for disposing of the separated liquids responsibly.
Understanding the Principles of Separation
The fundamental principle behind separating gasoline and water relies on their differing densities. Water has a higher density than gasoline, meaning it's heavier. This density difference allows us to exploit several separation techniques effectively. The techniques we'll discuss primarily leverage this density difference, exploiting the tendency of the heavier liquid (water) to settle below the lighter liquid (gasoline).
Methods for Separating Gasoline and Water
Several methods effectively separate gasoline and water. The choice depends on the scale of the separation task and the desired purity of the resulting gasoline.
1. Separation Using a Separatory Funnel (Laboratory Scale)
This is the most common and accurate method used in laboratories or for smaller volumes of gasoline-water mixtures. A separatory funnel is a piece of laboratory glassware with a stopcock at the bottom.
Steps:
- Careful Transfer: Gently pour the gasoline-water mixture into the separatory funnel. Avoid splashing to minimize the risk of spills.
- Allow Settling: Let the mixture stand undisturbed for a sufficient time to allow the two liquids to separate completely. The water will settle to the bottom, forming a distinct layer below the gasoline. This settling time will vary based on the volume; smaller volumes may only need a few minutes, while larger volumes could require longer.
- Drainage: Open the stopcock slowly, draining the water layer into a separate container. Be careful to stop draining just before the gasoline layer begins to flow.
- Collection: Collect the separated gasoline layer into another container. Again, proceed carefully to prevent contamination.
- Safety: Always wear appropriate safety gear, including gloves and eye protection, when handling gasoline. Perform the separation in a well-ventilated area.
Advantages: High accuracy, relatively simple, good for small to medium volumes.
Disadvantages: Requires specialized equipment, not suitable for large-scale separation.
2. Decantation (Small-Scale Separation)
Decantation is a simple method for separating immiscible liquids with a significant density difference. This is a good option for small-scale spills.
Steps:
- Allow Settling: Let the mixture sit undisturbed for a considerable time to ensure complete separation of the layers.
- Careful Pouring: Gently pour off the top layer (gasoline) into a separate container, leaving the water behind.
- Residual Removal: Some water will remain. This can be minimized by tilting the container at an angle during pouring, slowing down the pouring rate.
Advantages: Easy, requires no specialized equipment.
Disadvantages: Less accurate than using a separatory funnel, not suitable for larger volumes, and not as effective in separating smaller amounts of water.
3. Siphoning (Medium-Scale Separation)
Siphoning can be used for slightly larger quantities of the gasoline-water mixture compared to decantation.
Steps:
- Settling: Allow the mixture to settle completely.
- Siphon Setup: Carefully position a siphon tube (a flexible tube) into the gasoline layer, ensuring it does not reach the bottom layer.
- Start the Siphon: Initiate the siphon by creating suction at the output end. The gasoline will flow through the tube into a separate container.
- Control the Flow: Watch carefully to stop before drawing any water.
Advantages: Suitable for slightly larger volumes than decantation.
Disadvantages: Requires more attention to detail than decantation, less accurate than the separatory funnel.
4. Gravity Separation (Large-Scale Separation)
This method is applicable to larger volumes of gasoline-water mixtures, often used in industrial settings. The process involves allowing gravity to fully separate the liquids over a longer duration. It may involve the use of settling tanks.
Steps:
- Transfer: Transfer the gasoline-water mixture into a large settling tank.
- Settling: The tank is designed to allow sufficient time for complete separation.
- Drainage: The water layer is drained from the bottom of the tank using valves.
- Gasoline Removal: The gasoline layer is then removed from the top of the tank.
Advantages: Suitable for large-scale operations.
Disadvantages: Requires specialized equipment and infrastructure; settling times may be significantly longer.
Safety Precautions
Gasoline is a highly flammable and volatile substance. Always prioritize safety when handling gasoline-water mixtures:
- Ventilation: Work in a well-ventilated area to prevent the buildup of flammable vapors.
- Fire Safety: Keep away from open flames, sparks, and sources of ignition.
- Personal Protective Equipment (PPE): Always wear appropriate safety gear, including gloves, eye protection, and closed-toe shoes.
- Spill Containment: If a spill occurs, immediately contain the spill using absorbent materials.
- Disposal: Dispose of separated liquids responsibly. Gasoline should be handled according to local regulations, often involving recycling or specialized disposal facilities. Never pour gasoline down drains or into the environment.
- Awareness: Be mindful of potential health hazards associated with gasoline inhalation or skin contact.
Choosing the Right Method
The most appropriate method for separating gasoline and water hinges on several factors:
- Volume: For small volumes (e.g., small spills), decantation is often sufficient. For medium volumes, a separatory funnel or siphoning is preferable. Large-scale separation necessitates gravity separation methods utilizing settling tanks.
- Purity Requirements: If high purity gasoline is essential, a separatory funnel is recommended.
- Available Equipment: Decantation requires no special equipment, while separatory funnels, siphoning, and large-scale gravity separation require specific tools and infrastructure.
Responsible Disposal
Proper disposal of separated gasoline and water is crucial for environmental protection and safety. Never pour gasoline down drains or into the environment. Contact your local waste management authority or a hazardous waste disposal facility to determine the proper disposal method for gasoline in your area. They can provide guidance on safe handling and disposal procedures. Water separated from the gasoline may still contain trace amounts of contaminants and should be disposed of accordingly, following local regulations.
Conclusion
Separating gasoline and water is achievable using various techniques, ranging from simple decantation to more sophisticated methods like gravity separation. Choosing the right method depends on the scale of the operation and desired purity level. The process, however, necessitates stringent adherence to safety protocols to mitigate the risks associated with handling gasoline, a highly flammable and volatile substance. Finally, remember that responsible disposal of separated liquids is paramount for environmental protection. Always follow local regulations and guidelines to ensure safe and appropriate disposal.
Latest Posts
Latest Posts
-
What Type Of Joint Is Between The Sternum And Rib
Mar 19, 2025
-
Receptacle Is Part Of The Four Whorls
Mar 19, 2025
-
The Bending Of Waves Around A Barrier
Mar 19, 2025
-
A Surveyor Measures The Distance Across A Straight River
Mar 19, 2025
-
Fluid Pressure Against A Wall Or Cell Membranes Is Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Separate Gasoline And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
