How To Find Mole Fraction From Molality
News Leon
Apr 06, 2025 · 5 min read
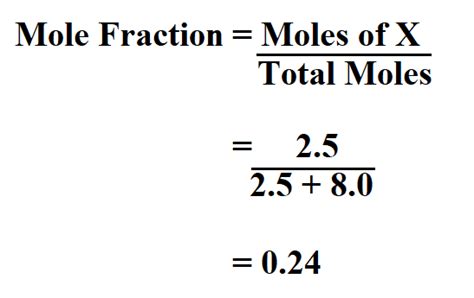
Table of Contents
How to Find Mole Fraction from Molality: A Comprehensive Guide
Determining the mole fraction of a solution is crucial in various chemical and engineering applications. While molality (moles of solute per kilogram of solvent) is a readily available concentration unit, it doesn't directly translate to mole fraction (moles of a component divided by total moles in the solution). This comprehensive guide will walk you through the process of calculating mole fraction from molality, covering different scenarios and offering practical examples.
Understanding the Fundamentals: Molality vs. Mole Fraction
Before delving into the calculations, let's refresh our understanding of molality and mole fraction:
Molality (m): Represents the number of moles of solute dissolved per kilogram of solvent. It's denoted as:
m = moles of solute / kilograms of solvent
Mole Fraction (χ): Represents the ratio of the number of moles of a particular component (solute or solvent) to the total number of moles in the solution. For a component 'i', it's calculated as:
χi = moles of component i / total moles in solution
The key difference lies in the denominator: molality uses the mass of the solvent, while mole fraction uses the total moles of all components (solute + solvent). This distinction necessitates a conversion process.
Calculating Mole Fraction from Molality: A Step-by-Step Approach
The conversion from molality to mole fraction involves several steps:
Step 1: Define the System
Clearly identify the solute and the solvent. Knowing the identity of each component is crucial for determining their molar masses. Let's consider a solution where:
- Solute: Substance A (molar mass = MA g/mol)
- Solvent: Substance B (molar mass = MB g/mol)
- Molality of the solution: 'm' mol/kg
Step 2: Assume a Basis
To simplify calculations, it's helpful to assume a basis. A common basis is to assume you have 1 kg of solvent (Substance B). This means:
- Kilograms of solvent (B) = 1 kg
Step 3: Calculate Moles of Solute (A)
Using the definition of molality, we can calculate the moles of solute (A):
- Moles of solute (A) = molality (m) * kilograms of solvent = m * 1 kg = m moles
Step 4: Calculate Moles of Solvent (B)
Convert the mass of the solvent (1 kg) into moles using its molar mass (MB):
- Moles of solvent (B) = (mass of solvent in grams) / (molar mass of solvent) = (1000 g) / (MB g/mol) = 1000/MB moles
Step 5: Calculate Total Moles
Determine the total number of moles in the solution by adding the moles of solute and solvent:
- Total moles = moles of solute (A) + moles of solvent (B) = m + 1000/MB moles
Step 6: Calculate Mole Fraction of Solute (A) and Solvent (B)
Finally, calculate the mole fraction of each component using the formula defined earlier:
-
Mole fraction of solute (A): χA = moles of A / total moles = m / (m + 1000/MB)
-
Mole fraction of solvent (B): χB = moles of B / total moles = (1000/MB) / (m + 1000/MB)
Important Note: The sum of the mole fractions of all components in a solution should always equal 1 (χA + χB = 1). This serves as a valuable check for your calculations.
Illustrative Examples
Let's illustrate the process with a few examples:
Example 1: Aqueous Solution of Sodium Chloride (NaCl)
Consider a 0.5 mol/kg aqueous solution of NaCl. The molar mass of water (solvent) is approximately 18.015 g/mol.
- Solute: NaCl
- Solvent: H₂O
- Molality (m): 0.5 mol/kg
- Molar mass of H₂O (MB): 18.015 g/mol
Following the steps outlined above:
- Moles of NaCl (A) = 0.5 moles
- Moles of H₂O (B) = 1000 g / 18.015 g/mol ≈ 55.51 moles
- Total moles = 0.5 + 55.51 = 56.01 moles
- Mole fraction of NaCl (χA) = 0.5 / 56.01 ≈ 0.0089
- Mole fraction of H₂O (χB) = 55.51 / 56.01 ≈ 0.9911
Example 2: Solution of Benzene in Toluene
Let's consider a solution of benzene (solute) in toluene (solvent) with a molality of 2.0 mol/kg. The molar mass of toluene is approximately 92.14 g/mol.
- Solute: Benzene
- Solvent: Toluene
- Molality (m): 2.0 mol/kg
- Molar mass of Toluene (MB): 92.14 g/mol
Following the steps:
- Moles of Benzene (A) = 2.0 moles
- Moles of Toluene (B) = 1000 g / 92.14 g/mol ≈ 10.85 moles
- Total moles = 2.0 + 10.85 = 12.85 moles
- Mole fraction of Benzene (χA) = 2.0 / 12.85 ≈ 0.156
- Mole fraction of Toluene (χB) = 10.85 / 12.85 ≈ 0.844
Handling Solutions with Multiple Solutes
The method can be extended to solutions containing multiple solutes. The approach remains similar: calculate the moles of each solute, the moles of the solvent, and then the total moles. The mole fraction of each component is then calculated individually using the same formula.
For instance, if you have a solution with solutes A and C, you will calculate:
- Moles of A
- Moles of C
- Moles of solvent B
- Total moles = Moles of A + Moles of C + Moles of B
Then, the mole fraction for each component is calculated using:
- χA = Moles of A / Total moles
- χC = Moles of C / Total moles
- χB = Moles of B / Total moles
Advanced Considerations and Potential Pitfalls
-
Non-ideal Solutions: The calculations presented here assume ideal solutions, where interactions between solute and solvent molecules are negligible. In non-ideal solutions, deviations from ideality can affect the relationship between molality and mole fraction. More complex models might be needed for accurate calculations in such cases.
-
Concentration Units: Ensure consistency in units. Always use consistent units throughout your calculations (moles, kilograms, grams).
-
Significant Figures: Pay attention to significant figures in your calculations. The final answer should reflect the precision of the input data.
-
Molar Mass Accuracy: Using accurate molar masses from reliable sources is essential for achieving accurate results.
Conclusion
Converting molality to mole fraction is a straightforward yet essential calculation in chemistry and related fields. By understanding the fundamental principles and following the step-by-step approach outlined in this guide, you can confidently perform these conversions for a wide range of solutions. Remember to always double-check your calculations and consider the limitations of the ideal solution assumption, especially when dealing with concentrated solutions or those exhibiting significant intermolecular interactions. This detailed guide provides the foundational knowledge necessary for accurate and efficient conversion between these crucial concentration units.
Latest Posts
Latest Posts
-
What Is The Final Product Of The Calvin Cycle
Apr 09, 2025
-
Write A Letter For Increment Of Salary
Apr 09, 2025
-
A Material That Does Not Conduct Heat Well
Apr 09, 2025
-
Is Air A Compound Element Or Mixture
Apr 09, 2025
-
Equipotential Surfaces Associated With An Electric Dipole Are
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How To Find Mole Fraction From Molality . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.