How To Calculate Percentage Of Ionic Character
News Leon
Mar 22, 2025 · 5 min read
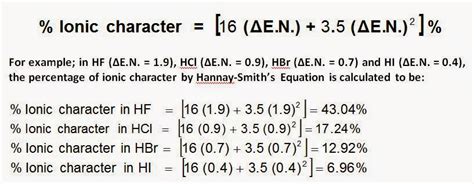
Table of Contents
How to Calculate the Percentage of Ionic Character: A Comprehensive Guide
Determining the percentage ionic character of a chemical bond is crucial in understanding the properties and behavior of molecules. While perfectly ionic or perfectly covalent bonds are theoretical extremes, most real-world bonds fall somewhere on a spectrum between these two ideals. This article provides a detailed explanation of how to calculate the percentage ionic character, delving into the underlying principles and offering practical examples.
Understanding the Nature of Chemical Bonds
Before we dive into calculations, let's establish a firm understanding of the nature of chemical bonds. Chemical bonds are the forces that hold atoms together in molecules. These bonds arise from the electrostatic attraction between atoms, primarily driven by the interaction of their valence electrons. Two main types of bonds exist:
Covalent Bonds
Covalent bonds involve the sharing of electrons between atoms. This sharing typically occurs between atoms with similar electronegativities, meaning they have a similar pull on the shared electrons. Examples include bonds between two hydrogen atoms (H₂), or between carbon and hydrogen atoms (in methane, CH₄). The more equally the electrons are shared, the more purely covalent the bond is considered.
Ionic Bonds
Ionic bonds, in contrast, involve the transfer of electrons from one atom to another. This transfer typically occurs between atoms with significantly different electronegativities. The atom with higher electronegativity attracts the electron(s) more strongly, gaining a negative charge (anion), while the atom losing the electron(s) becomes positively charged (cation). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Examples include the bond between sodium (Na) and chlorine (Cl) in sodium chloride (NaCl), or between magnesium (Mg) and oxygen (O) in magnesium oxide (MgO).
Electronegativity: The Key to Ionic Character
The key to understanding and calculating the percentage ionic character of a bond lies in the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Higher electronegativity means a stronger pull on shared electrons. Various scales exist to quantify electronegativity, the most common being the Pauling scale. This scale assigns arbitrary values to elements, with fluorine (F) having the highest electronegativity (4.0).
Methods for Calculating Percentage Ionic Character
Several methods exist to estimate the percentage ionic character of a bond. The most commonly used methods are:
1. Using the Electronegativity Difference
This method relies on the difference in electronegativity (Δχ) between the two atoms involved in the bond. The larger the difference, the greater the ionic character. A simple formula often used is:
% Ionic Character ≈ 16|Δχ| + 3.5|Δχ|²
Where:
- Δχ = |χₐ - χբ| (the absolute difference between the electronegativities of atoms a and b)
- χₐ and χբ are the electronegativities of atoms a and b respectively, obtained from a standard electronegativity table (usually Pauling scale).
This formula provides a reasonable estimate, particularly for smaller electronegativity differences. However, it becomes less accurate for very large differences.
Example:
Let's calculate the percentage ionic character of the bond in NaCl.
- Electronegativity of Na (χNa) ≈ 0.93
- Electronegativity of Cl (χCl) ≈ 3.16
- Δχ = |3.16 - 0.93| = 2.23
% Ionic Character ≈ 16|2.23| + 3.5|2.23|² ≈ 35.68 + 17.36 ≈ 53%
This calculation suggests that the NaCl bond has approximately 53% ionic character.
2. Using the Dipole Moment
A more sophisticated approach involves using the dipole moment (μ) of the molecule. The dipole moment is a measure of the separation of positive and negative charges within a molecule. A larger dipole moment indicates a greater degree of charge separation, and thus, higher ionic character.
The dipole moment (μ) can be experimentally determined or calculated using computational methods. The percentage ionic character can then be estimated by comparing the observed dipole moment to the dipole moment expected for a purely ionic bond. However, this method is more complex and requires specialized knowledge.
3. Considering Other Factors
It's important to note that electronegativity difference is not the sole determinant of ionic character. Other factors can influence the percentage ionic character, including:
- Size of the atoms: Larger atoms are more easily polarized, leading to a higher degree of covalent character in seemingly ionic bonds.
- Crystal structure: The arrangement of ions in a crystal lattice can affect the overall electrostatic interactions and hence the percentage ionic character.
- Environmental factors: Solvent effects and pressure can also influence the degree of ionic character.
Limitations of Percentage Ionic Character Calculations
While these methods provide valuable estimates, it's crucial to recognize their limitations:
- Approximations: The formulas used are approximations and may not accurately reflect the true nature of the bond in all cases.
- Simplified Models: These calculations simplify complex interactions between electrons and nuclei. They don't account for all factors influencing bond character.
- Continuum, not Categories: The percentage ionic character represents a position along a continuum between purely covalent and purely ionic, not distinct categories.
Practical Applications
Understanding the percentage ionic character is crucial in various fields, including:
- Predicting chemical properties: The ionic character affects melting points, boiling points, solubility, and reactivity.
- Material science: The design and synthesis of materials with specific properties often depend on controlling the ionic character of bonds.
- Biochemistry: Understanding bond character is essential in understanding the interactions between biomolecules.
- Catalysis: The ionic character of bonds can influence the catalytic activity of materials.
Conclusion
Calculating the percentage ionic character of a chemical bond provides valuable insights into the nature of the bond and the properties of the molecule. While various methods exist, each has its limitations. Using electronegativity difference provides a relatively simple yet useful estimate. The choice of method depends on the available data and the desired level of accuracy. Remember that the percentage ionic character should be viewed as an approximation rather than an absolute value, reflecting the complex interplay of forces within the molecule. Further research and advanced computational methods offer a more comprehensive understanding of bond character. However, the methods described above offer a foundational understanding suitable for many applications.
Latest Posts
Latest Posts
-
Which Of The Following Organisms Is A Prokaryote
Mar 22, 2025
-
How Many Kg Is 5000 G
Mar 22, 2025
-
How Many Vertices On A Sphere
Mar 22, 2025
-
The Largest Gulf In The World
Mar 22, 2025
-
Advertising Is A Form Of Paid And Non Personal Promotion
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Percentage Of Ionic Character . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
