How To Calculate Boiling Point From Entropy And Enthalpy
News Leon
Mar 20, 2025 · 5 min read
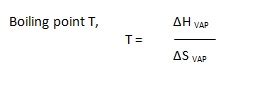
Table of Contents
How to Calculate Boiling Point from Entropy and Enthalpy
Boiling point, a fundamental physical property, signifies the temperature at which a substance transitions from its liquid to gaseous phase at a given pressure. While experimentally determining boiling point is straightforward, understanding its thermodynamic underpinnings provides deeper insights into the substance's molecular behavior. This article delves into the theoretical calculation of boiling point using entropy and enthalpy, exploring the underlying principles and offering practical approaches for various scenarios.
Understanding the Thermodynamic Principles
The boiling process is governed by the interplay of enthalpy (ΔH) and entropy (ΔS). Enthalpy represents the heat absorbed during the phase transition, while entropy reflects the increase in disorder or randomness as the liquid molecules transition to the more disordered gaseous state. At the boiling point, the Gibbs free energy (ΔG), a thermodynamic potential that determines the spontaneity of a process, is zero.
The relationship between Gibbs free energy, enthalpy, and entropy is expressed by the following equation:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs free energy
- ΔH is the change in enthalpy (heat of vaporization)
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy
At the boiling point (Tb), the system is in equilibrium between the liquid and gas phases, implying that ΔG = 0. Therefore, the equation simplifies to:
0 = ΔH - TbΔS
Solving for the boiling point (Tb), we obtain:
Tb = ΔH / ΔS
This equation forms the basis for calculating the boiling point using enthalpy and entropy data.
Obtaining Enthalpy (ΔH) and Entropy (ΔS) Data
Before we proceed with the calculation, obtaining accurate values for enthalpy of vaporization (ΔHvap) and entropy of vaporization (ΔSvap) is crucial. These values are typically obtained through experimental methods, but estimations can be made using various correlations and approximations.
1. Experimental Determination:
The most reliable approach involves experimental techniques like calorimetry. Calorimetry measures the heat absorbed or released during a process. In the context of boiling point determination, calorimetric measurements would quantify the heat required to vaporize a known mass of the substance at its boiling point, providing a direct measure of ΔHvap. Similarly, entropy changes can be determined from experimental data by measuring heat capacities and integrating over temperature ranges.
2. Using Trouton's Rule:
When experimental data is unavailable, Trouton's rule provides a reasonable approximation for the entropy of vaporization:
ΔSvap ≈ 88 J/(mol·K)
Trouton's rule states that the entropy of vaporization is approximately constant for many liquids, around 88 J/(mol·K). However, this rule is an approximation and deviations can occur, especially for substances with strong intermolecular forces (like hydrogen bonding) or those with low boiling points. For more accurate estimations, refined versions of Trouton's rule exist that incorporate factors like critical temperature and pressure.
3. Using Clausius-Clapeyron Equation:
The Clausius-Clapeyron equation relates the vapor pressure of a substance to its temperature and enthalpy of vaporization:
ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)
Where:
- P1 and P2 are vapor pressures at temperatures T1 and T2 respectively.
- R is the ideal gas constant.
By measuring vapor pressure at two different temperatures, one can determine ΔHvap. Once ΔHvap is known, ΔSvap can be calculated using the relationship at the boiling point:
ΔSvap = ΔHvap/Tb (at normal boiling point, where pressure is 1 atm)
This method is particularly useful when experimental data at the boiling point itself is difficult to obtain.
4. Computational Methods:
Advanced computational methods using molecular dynamics simulations and quantum chemical calculations can provide accurate estimates of enthalpy and entropy of vaporization. These methods require significant computational resources and expertise but offer a powerful alternative to experimental approaches, especially for substances difficult to handle experimentally.
Calculating Boiling Point: A Step-by-Step Guide
Once the enthalpy (ΔHvap) and entropy (ΔSvap) of vaporization are determined (through experimental measurements or estimations using methods described above), calculating the boiling point becomes straightforward.
1. Convert Units:
Ensure that all units are consistent. ΔHvap should be in Joules per mole (J/mol), ΔSvap in Joules per mole per Kelvin (J/(mol·K)), and the desired boiling point will be in Kelvin (K).
2. Apply the Formula:
Use the formula derived earlier:
Tb = ΔHvap / ΔSvap
3. Convert to Celsius (Optional):
If a Celsius boiling point is required, subtract 273.15 from the Kelvin value:
Tb (°C) = Tb (K) - 273.15
Illustrative Example
Let's assume that for a particular substance, the experimentally determined enthalpy of vaporization (ΔHvap) is 30,000 J/mol, and the entropy of vaporization (ΔSvap) is 100 J/(mol·K).
Using the formula:
Tb = ΔHvap / ΔSvap = 30,000 J/mol / 100 J/(mol·K) = 300 K
Converting to Celsius:
Tb (°C) = 300 K - 273.15 = 26.85 °C
Therefore, the calculated boiling point of this substance is approximately 26.85 °C.
Limitations and Considerations
While the method described provides a powerful theoretical approach for calculating boiling point, several limitations need consideration:
- Ideal Behavior Assumption: The calculations often assume ideal behavior for both the liquid and gas phases. Deviations from ideal behavior, particularly at higher pressures or for substances with strong intermolecular interactions, can lead to inaccuracies.
- Temperature Dependence: Enthalpy and entropy of vaporization are themselves temperature-dependent. The values used in the calculation should ideally be those corresponding to the boiling point temperature. Using values at a significantly different temperature will introduce errors.
- Accuracy of Input Data: The accuracy of the calculated boiling point is directly linked to the accuracy of the input data (ΔHvap and ΔSvap). Experimental errors or limitations in estimation techniques can significantly impact the result.
- Association and Dissociation: The method assumes that the substance does not undergo significant association (like dimerization) in the liquid phase or dissociation in the gas phase. If such processes occur, the calculated boiling point might deviate from the actual value.
Conclusion
Calculating boiling point from entropy and enthalpy offers a valuable theoretical understanding of this fundamental physical property. The approach, while reliant on accurate thermodynamic data, provides a powerful tool for predicting boiling points, especially when experimental determination is challenging. By carefully considering the limitations and employing appropriate estimation techniques, one can obtain reasonably accurate results, bridging the gap between theoretical understanding and practical applications. Remember that the accuracy is heavily dependent on the accuracy of the enthalpy and entropy values used, necessitating careful consideration of the data sources and potential experimental uncertainties. Utilizing multiple methods for obtaining ΔH and ΔS and comparing the results offers a more robust approach.
Latest Posts
Latest Posts
-
What Do You Call People From France
Mar 28, 2025
-
Match The Label To The Correct Structure On The Chloroplast
Mar 28, 2025
-
Longest Cell In The Human Body
Mar 28, 2025
-
A Part Of A Line With Two Endpoints
Mar 28, 2025
-
Choose The Correct Statement From The Following
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Boiling Point From Entropy And Enthalpy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
