How Many Valence Electrons In Lithium
News Leon
Mar 23, 2025 · 6 min read
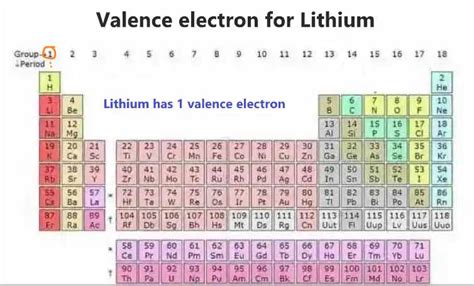
Table of Contents
How Many Valence Electrons Does Lithium Have? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, plays a fascinating role in chemistry and technology. Understanding its electronic structure, specifically the number of valence electrons, is crucial to grasping its reactivity and unique properties. This article will explore the concept of valence electrons, delve into the electronic configuration of lithium, explain why it possesses the number of valence electrons it does, and finally, discuss the implications of this characteristic in its chemical behavior.
Understanding Valence Electrons: The Key to Reactivity
Before we dive into lithium's specific case, let's establish a firm understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the furthest from the nucleus and, therefore, experience the weakest attraction to it. This weaker attraction makes them highly susceptible to interaction with other atoms, leading to the formation of chemical bonds. The number of valence electrons an atom possesses dictates its bonding capacity and chemical reactivity. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their full outermost electron shells.
The Significance of the Octet Rule
The octet rule, while not universally applicable, provides a helpful guideline for understanding the reactivity of many elements. The rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell containing eight electrons (a stable octet), mimicking the electron configuration of noble gases. This stable configuration provides significant stability, minimizing the atom's energy state. Exceptions to the octet rule exist, particularly with elements in the second period and beyond, but it remains a valuable tool for predicting chemical behavior.
Delving into Lithium's Electronic Structure
Lithium (Li), with an atomic number of 3, has three protons in its nucleus and, therefore, three electrons surrounding it. To understand the distribution of these electrons, we must consider the principles of electron configuration. Electrons occupy specific energy levels or shells, with each shell capable of holding a certain maximum number of electrons. The closer a shell is to the nucleus, the lower its energy level.
Lithium's Electron Configuration: A Step-by-Step Breakdown
The electron configuration of lithium is 1s²2s¹. Let's break this down:
- 1s²: This indicates that two electrons occupy the first energy level (n=1), specifically the s subshell. The s subshell can hold a maximum of two electrons.
- 2s¹: This signifies that one electron resides in the second energy level (n=2), also in the s subshell.
Identifying Lithium's Valence Electrons
Since valence electrons are those in the outermost shell, we can easily identify them in lithium's configuration. The outermost shell for lithium is the second energy level (n=2), containing only one electron in the 2s orbital. Therefore, lithium has only one valence electron.
Why Only One Valence Electron? A Deeper Look at Atomic Structure
The reason lithium possesses just one valence electron stems directly from its electron configuration and the filling of atomic orbitals. Electrons fill orbitals according to the Aufbau principle, which states that electrons occupy the lowest available energy levels first. The 1s orbital, being the lowest in energy, fills first with two electrons (due to the Pauli Exclusion Principle, which states that each orbital can hold a maximum of two electrons with opposite spins). The next available energy level is the 2s orbital, which receives the remaining electron, resulting in lithium's characteristic 1s²2s¹ configuration.
The Role of Quantum Numbers
The location and energy of electrons within an atom are described by a set of four quantum numbers:
- Principal quantum number (n): Represents the energy level or shell (n = 1, 2, 3...).
- Azimuthal quantum number (l): Specifies the subshell (l = 0 for s, 1 for p, 2 for d, etc.).
- Magnetic quantum number (ml): Determines the orientation of the orbital within the subshell.
- Spin quantum number (ms): Represents the intrinsic angular momentum of the electron (ms = +1/2 or -1/2).
These quantum numbers define the specific orbital each electron occupies within the lithium atom, ultimately contributing to the determination of its single valence electron.
Implications of Lithium's Single Valence Electron
The presence of only one valence electron profoundly influences lithium's chemical and physical properties:
High Reactivity: A Lone Electron's Quest for Stability
Lithium's single valence electron makes it highly reactive. To achieve a stable octet (or, in this case, a duet, resembling helium), lithium readily loses this electron to form a +1 cation (Li⁺). This electron loss is energetically favorable, leading to the formation of ionic compounds with other elements that readily accept electrons (like halogens).
Low Ionization Energy: Easy Electron Loss
The ease with which lithium loses its valence electron is reflected in its low ionization energy. Ionization energy is the energy required to remove an electron from an atom. Because lithium's outermost electron is weakly held, its ionization energy is relatively low, making it easier to form ions and participate in chemical reactions.
Metallic Bonding and Properties: Sharing Electrons in the Metallic Sea
Lithium's single valence electron also contributes to its metallic nature. In solid lithium, these valence electrons are delocalized, forming a "sea" of electrons that move freely throughout the metal lattice. This "sea" of electrons provides strong metallic bonding, resulting in properties like good electrical and thermal conductivity, malleability, and ductility.
Applications Leveraging Lithium's Unique Properties
Lithium's unique properties, directly attributable to its single valence electron, make it a valuable element in various applications, including:
- Lithium-ion batteries: Lithium's ability to readily lose and gain electrons makes it ideal for use in rechargeable batteries, powering many portable electronic devices and electric vehicles.
- Lubricants: Lithium-based greases are widely used as lubricants due to their excellent high-temperature stability and resistance to water.
- Aluminum alloys: Small additions of lithium to aluminum alloys improve their strength and stiffness.
- Glass and ceramics: Lithium compounds are incorporated into glass and ceramic formulations to improve their properties.
- Medicine: Lithium salts have therapeutic applications in treating bipolar disorder.
Conclusion: A Single Electron with a Big Impact
In conclusion, lithium, with its single valence electron, showcases the profound impact of atomic structure on chemical behavior and material properties. Its relatively simple electron configuration allows us to understand its high reactivity, low ionization energy, and unique metallic bonding characteristics. These properties, in turn, lead to its wide range of applications across diverse fields. Understanding the fundamental principle of valence electrons and its impact on elemental behavior remains essential to mastering the realm of chemistry and materials science. The seemingly simple fact that lithium possesses only one valence electron is the key to unlocking a wealth of its fascinating and impactful properties.
Latest Posts
Latest Posts
-
What Is The Difference Between A Cell And Tissue
Mar 25, 2025
-
Integral 2xdx From 10 To 13
Mar 25, 2025
-
What Is The Condensation Point Of Water In Celsius
Mar 25, 2025
-
Which Poem Are These Lines From
Mar 25, 2025
-
What Is Not A Subatomic Particle
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
