How Many Subshells Are In The N 4 Shell
News Leon
Mar 23, 2025 · 5 min read
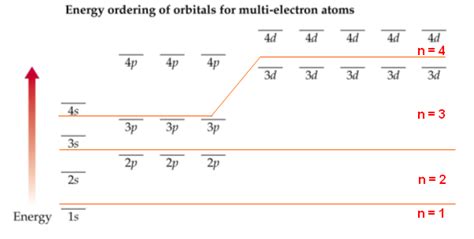
Table of Contents
How Many Subshells Are in the n=4 Shell? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to grasping the behavior of atoms and molecules. A key element of this understanding involves knowing how many subshells exist within each principal energy level (shell), denoted by the principal quantum number, n. This article delves into the specifics of the n=4 shell, exploring the number of subshells, their orbital types, and the implications for electron occupancy.
Understanding Quantum Numbers and Shells
Before we delve into the n=4 shell, let's review the basics. Electrons within an atom occupy specific energy levels, often visualized as shells surrounding the nucleus. These shells are characterized by the principal quantum number, n, which can take on positive integer values (1, 2, 3, 4...). The higher the value of n, the greater the energy level and the further the electrons are from the nucleus.
Within each shell, electrons further occupy subshells, which are defined by the azimuthal quantum number, l. The value of l can range from 0 to n-1. Each value of l corresponds to a specific subshell type:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex, multi-lobed shape)
- l = 3: f subshell (even more complex shape)
The number of subshells within a given shell, therefore, is equal to the value of n.
The n=4 Shell: Unveiling its Subshells
Now, let's focus on the n=4 shell. Since n=4, the possible values of l are 0, 1, 2, and 3. This means the n=4 shell contains four subshells:
- 4s subshell (l = 0): This subshell has a single orbital, capable of holding a maximum of two electrons (due to the Pauli Exclusion Principle).
- 4p subshell (l = 1): This subshell has three orbitals (px, py, pz), each capable of holding two electrons. Therefore, the 4p subshell can hold a maximum of six electrons.
- 4d subshell (l = 2): This subshell possesses five orbitals, allowing it to accommodate a maximum of ten electrons.
- 4f subshell (l = 3): This is the most complex subshell in the n=4 shell, containing seven orbitals and a maximum electron capacity of fourteen electrons.
Total Number of Subshells and Electrons in the n=4 Shell
In summary, the n=4 shell contains four subshells: 4s, 4p, 4d, and 4f. The total number of electrons that can be accommodated within the n=4 shell is the sum of the maximum electron capacity of each subshell: 2 (4s) + 6 (4p) + 10 (4d) + 14 (4f) = 32 electrons.
Electron Configuration and the n=4 Shell
The electron configuration of an atom describes how electrons are distributed among the various shells and subshells. The filling of these shells and subshells follows specific rules, primarily the Aufbau principle (electrons fill lower energy levels first), Hund's rule (electrons fill orbitals individually before pairing), and the Pauli Exclusion Principle (no two electrons can have the same four quantum numbers).
Elements with electrons in the n=4 shell are found in the fourth period (row) of the periodic table. As you move across this period, electrons progressively fill the 4s, 4p, and 3d subshells. Note the 3d subshell fills after the 4s subshell due to slight variations in energy levels. The 4f subshell starts filling much later, in the lanthanide series (rare earth elements).
Practical Applications and Importance
Understanding the number of subshells and electron capacity of the n=4 shell is critical in numerous areas:
-
Predicting Chemical Properties: The electron configuration, determined by the filling of subshells, directly influences an element's chemical behavior and reactivity. The outermost electrons (valence electrons), often found in the n=4 shell for heavier elements, are primarily responsible for chemical bonding.
-
Spectroscopy: The transitions of electrons between different subshells within the n=4 shell (and other shells) produce characteristic spectral lines used in various analytical techniques. This allows us to identify and quantify elements.
-
Materials Science: Understanding electronic structures is crucial in designing and synthesizing novel materials with specific properties. The behavior of electrons in the n=4 shell impacts the conductivity, magnetism, and other material characteristics.
-
Nuclear Chemistry: While primarily focused on the nucleus, nuclear processes often affect electron configurations. Understanding the n=4 shell's structure is relevant when considering radioactive decay and its impact on electronic structure.
-
Quantum Mechanics and Computational Chemistry: The theoretical framework of quantum mechanics relies heavily on understanding the various quantum numbers and electron configurations. Advanced computational models used to study molecules and materials use this knowledge as a basis.
Beyond the Basics: Orbital Shapes and Energy Levels
While we've established the number of subshells, it's beneficial to delve a bit further into the nuances:
-
Orbital Shapes: The s, p, d, and f orbitals possess unique shapes that influence chemical bonding and interactions. The 4s orbital is spherical, the 4p orbitals are dumbbell-shaped, and the 4d and 4f orbitals have even more complex shapes. These shapes directly affect the spatial distribution of electrons and their interactions with other atoms.
-
Energy Levels: Although generally the energy level increases with the principal quantum number (n), there are subtle energy differences between subshells within the same shell, particularly in heavier atoms. This explains why the 4s subshell fills before the 3d subshell. The subtle energy differences are a consequence of shielding effects and inter-electron repulsion.
-
Shielding Effects: Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect alters the effective nuclear charge experienced by the electrons, leading to variations in energy levels between subshells.
Conclusion: The Significance of the n=4 Shell
In conclusion, the n=4 shell houses four subshells: 4s, 4p, 4d, and 4f, capable of accommodating a total of 32 electrons. This knowledge is fundamental in understanding atomic structure, predicting chemical properties, and advancing our understanding of various scientific fields. The intricacies of electron configuration, including the subtleties of energy level differences and shielding effects, further enhance our grasp of atomic behavior and the properties of matter. The importance of the n=4 shell, and the principles governing electron distribution within it, underlines its central role in chemistry, physics, and materials science. Continued exploration of these fundamental concepts will undoubtedly lead to further breakthroughs in our understanding of the universe at its most fundamental level.
Latest Posts
Latest Posts
-
Animals That Can Grow Lungs After Being Born
Mar 25, 2025
-
The Ability To Respond To A Stimulus
Mar 25, 2025
-
What Is The Area Of Triangle Lmn
Mar 25, 2025
-
The Figure Shows A Circular Region Of Radius R
Mar 25, 2025
-
30 Is 60 Percent Of What Number
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Subshells Are In The N 4 Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
