How Many Stereoisomers Are Possible For
News Leon
Mar 16, 2025 · 5 min read
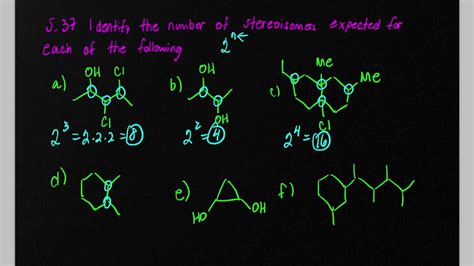
Table of Contents
Determining the Number of Stereoisomers: A Comprehensive Guide
Determining the number of possible stereoisomers for a molecule is a crucial aspect of organic chemistry, impacting fields like drug design, materials science, and biochemistry. Stereoisomers are molecules with the same molecular formula and connectivity but differ in the three-dimensional arrangement of their atoms. Understanding how to calculate the number of stereoisomers is essential for predicting a molecule's properties and behavior. This article will delve into various methods and considerations for calculating the number of possible stereoisomers, focusing on different types of stereoisomerism.
Understanding Stereoisomerism
Before delving into calculations, let's establish a solid understanding of the different types of stereoisomerism:
-
Enantiomers: These are non-superimposable mirror images of each other. They possess identical physical properties (except for the direction they rotate plane-polarized light) and react identically with achiral reagents. A classic example is the pair of enantiomers for lactic acid.
-
Diastereomers: These are stereoisomers that are not mirror images of each other. They have different physical properties and react differently with both chiral and achiral reagents. Diastereomers arise when a molecule has multiple chiral centers.
-
Cis-Trans Isomerism (Geometric Isomerism): This type of stereoisomerism occurs in molecules with restricted rotation, such as those containing double bonds or cyclic structures. Cis isomers have substituents on the same side of the double bond or ring, while trans isomers have substituents on opposite sides.
-
Meso Compounds: These are achiral molecules possessing chiral centers. They possess a plane of symmetry that renders the molecule superimposable on its mirror image, thus lacking optical activity.
Calculating the Number of Stereoisomers: The 2<sup>n</sup> Rule
The simplest way to estimate the maximum number of stereoisomers for a molecule containing n chiral centers is using the 2<sup>n</sup> rule. This rule assumes that each chiral center can exist in two configurations (R or S), and the total number of stereoisomers is the product of the possible configurations for each center. However, this rule has limitations:
-
Meso Compounds: The 2<sup>n</sup> rule overestimates the number of stereoisomers when meso compounds are present. Meso compounds are achiral despite having chiral centers, reducing the total number of distinct stereoisomers.
-
Cis-Trans Isomerism: The 2<sup>n</sup> rule doesn't directly account for cis-trans isomerism. If a molecule has both chiral centers and double bonds or rings, you need to consider both types of stereoisomerism separately and then multiply the possibilities.
Example: A molecule with three chiral centers would, according to the 2<sup>n</sup> rule, have a maximum of 2<sup>3</sup> = 8 stereoisomers. However, if one of the possible configurations leads to a meso compound, the actual number of distinct stereoisomers would be less than 8.
Advanced Techniques for Determining Stereoisomer Numbers
For complex molecules with multiple chiral centers and other sources of stereoisomerism, the 2<sup>n</sup> rule may be insufficient. More sophisticated techniques are needed:
-
Detailed Structural Analysis: Carefully examine the molecule's structure to identify all chiral centers and any possibilities for cis-trans isomerism. Draw out all possible configurations to visually check for duplicates or meso compounds. This method is time-consuming but ensures accuracy, especially for smaller molecules.
-
Symmetry Considerations: Recognizing planes of symmetry can significantly simplify the process. If a molecule possesses a plane of symmetry, it will be a meso compound, regardless of the number of chiral centers.
-
Computer-Aided Methods: Computational chemistry software packages can predict the number and types of stereoisomers for complex molecules. These programs use algorithms to generate all possible conformations and identify unique stereoisomers, considering factors like conformational flexibility and energy minima.
Case Studies: Applying the Principles
Let's illustrate the process with a couple of examples:
Example 1: 2,3-Dibromobutane
This molecule has two chiral centers (carbons 2 and 3). Using the 2<sup>n</sup> rule (n=2), we initially predict 2<sup>2</sup> = 4 stereoisomers. Drawing these structures, we find two pairs of enantiomers and no meso compounds. Therefore, there are four distinct stereoisomers: (2R,3R)-2,3-dibromobutane, (2S,3S)-2,3-dibromobutane, (2R,3S)-2,3-dibromobutane, and (2S,3R)-2,3-dibromobutane. (The last two are a pair of enantiomers).
Example 2: Tartaric Acid
Tartaric acid has two chiral centers. The 2<sup>n</sup> rule suggests 2<sup>2</sup> = 4 stereoisomers. However, one of the configurations results in a meso compound due to the presence of a plane of symmetry. Therefore, there are only three distinct stereoisomers: two enantiomers and one meso compound.
Example 3: A molecule with both chiral centers and a double bond
Consider a molecule with two chiral centers and one double bond capable of cis-trans isomerism. The 2<sup>n</sup> rule for the chiral centers gives 2<sup>2</sup> = 4 possibilities. The double bond adds another factor of 2 (cis and trans). Therefore, the total number of stereoisomers would be 4 x 2 = 8.
Conclusion: Navigating the Complexity of Stereoisomerism
Determining the number of stereoisomers for a molecule can range from straightforward to extremely complex, depending on the molecule's structure and the presence of various types of stereoisomerism. While the 2<sup>n</sup> rule provides a quick initial estimate, it's crucial to account for meso compounds and other forms of isomerism, using careful structural analysis or computational methods for accuracy. Understanding the principles presented here is fundamental to comprehending the properties and behavior of molecules in various chemical and biological contexts. A thorough understanding of stereoisomerism is crucial for advancements in numerous scientific fields, from pharmaceutical development to materials science. The ability to accurately predict and differentiate between stereoisomers is essential for successful research and innovation.
Latest Posts
Latest Posts
-
Oxidation Number Of Cr2 In K2cr2o7
Mar 16, 2025
-
How Many Diagonals Does A Octagon Have
Mar 16, 2025
-
Which Of The Following Is The Start Codon
Mar 16, 2025
-
How Many Meters In 200 Cm
Mar 16, 2025
-
6 Is 30 Percent Of What
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Stereoisomers Are Possible For . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
