How Many Protons Does Chloride Have
News Leon
Mar 17, 2025 · 5 min read
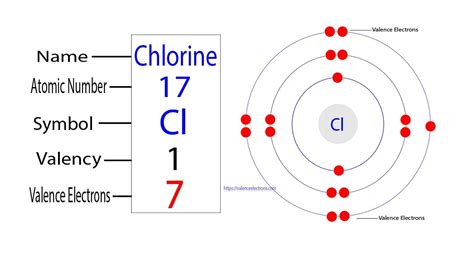
Table of Contents
How Many Protons Does Chloride Have? A Deep Dive into Atomic Structure and Isotopes
Understanding the fundamental building blocks of matter is crucial in various scientific fields. This article delves into the question: how many protons does chloride have? We'll explore the concept of atomic number, delve into the specifics of chlorine and its isotopes, and examine the implications of proton count on chemical behavior. We will also touch upon related concepts like electron configuration and ionic bonding, to provide a comprehensive understanding of this seemingly simple question.
Understanding Atomic Number and Protons
The number of protons in an atom's nucleus defines its atomic number, a fundamental property that uniquely identifies each element. This number is incredibly important because it dictates the element's chemical properties and its position on the periodic table. Every atom of a given element will always have the same number of protons. Changing the number of protons fundamentally changes the element itself. Adding or subtracting a proton transforms the atom into a different element entirely.
The Role of Protons in Atomic Structure
Protons, along with neutrons, reside in the atom's nucleus, forming the vast majority of its mass. Electrons, much lighter particles, orbit the nucleus in electron shells or energy levels. The positive charge of protons is balanced by the negative charge of electrons in a neutral atom. This balance of charges is essential for the atom's stability. The number of neutrons can vary within an element, leading to isotopes (discussed below), but the proton number remains constant.
Chlorine: Atomic Number 17
Chlorine (Cl), a highly reactive nonmetal, occupies the 17th position on the periodic table. This position directly reflects its atomic number. Therefore, the answer to our central question is: a chloride ion has 17 protons. It's crucial to differentiate between a chlorine atom and a chloride ion. A chlorine atom is neutral and has 17 protons and 17 electrons. A chloride ion (Cl⁻), however, has gained an extra electron to achieve a stable electron configuration, resulting in 17 protons and 18 electrons. The number of protons, however, remains unchanged.
Isotopes of Chlorine: Variations in Neutron Count
While the number of protons determines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Chlorine has two naturally occurring isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl).
-
Chlorine-35 (³⁵Cl): This isotope comprises about 76% of naturally occurring chlorine. It contains 17 protons and 18 neutrons (35 - 17 = 18).
-
Chlorine-37 (³⁷Cl): This isotope makes up the remaining ~24% of naturally occurring chlorine. It contains 17 protons and 20 neutrons (37 - 17 = 20).
The mass number (the superscript in the isotopic notation) represents the total number of protons and neutrons in the nucleus. It's important to remember that despite the variation in neutrons, both isotopes retain 17 protons, thus remaining chlorine atoms. The differing neutron numbers only slightly affect the atom's mass and some nuclear properties. Chemically, these isotopes behave virtually identically.
Implications of Proton Count: Chemical Behavior
The number of protons directly influences an atom's chemical behavior. The arrangement of electrons in energy levels, determined by the number of protons, dictates how an atom interacts with other atoms. Chlorine, with its seven valence electrons (electrons in the outermost shell), is highly reactive. It readily gains one electron to achieve a stable octet (eight electrons in its outermost shell), forming the chloride ion (Cl⁻). This tendency to gain an electron is a hallmark of its chemical behavior and is directly linked to its 17 protons.
Ionic Bonding and Chloride Ions
The formation of chloride ions through ionic bonding is a prime example of the importance of proton number. Chlorine's high electronegativity (its tendency to attract electrons) allows it to easily gain an electron from another atom, typically a metal, forming an ionic bond. The resulting ionic compound, such as sodium chloride (NaCl, common table salt), is held together by electrostatic forces between the positively charged sodium ion (Na⁺) and the negatively charged chloride ion (Cl⁻). This ionic bond is a direct consequence of chlorine's 17 protons and its drive to achieve a stable electron configuration.
Beyond the Basics: Exploring Related Concepts
Understanding the proton count of chloride opens the door to exploring more complex chemical concepts.
Electron Configuration
Chlorine's 17 electrons are arranged in specific energy levels according to the Aufbau principle and Hund's rule. Its electron configuration is 1s²2s²2p⁶3s²3p⁵. This configuration, directly related to its 17 protons, dictates its chemical reactivity and bonding behavior. The five electrons in the 3p subshell are the valence electrons, readily available for bonding.
Isotopic Abundance and Atomic Mass
The two isotopes of chlorine, ³⁵Cl and ³⁷Cl, exist in nature with different abundances. The weighted average of the masses of these isotopes, considering their relative abundance, gives chlorine's atomic mass, approximately 35.45 amu (atomic mass units). This average mass is a reflection of the isotopic composition and is a valuable piece of information in various chemical calculations.
Nuclear Chemistry and Radioisotopes
While ³⁵Cl and ³⁷Cl are stable isotopes, chlorine also possesses radioactive isotopes. These isotopes have unstable nuclei and undergo radioactive decay, emitting particles or energy to achieve stability. The study of radioactive isotopes is a vital area of nuclear chemistry with applications in various fields, including medicine and environmental science. The number of protons remains unchanged during radioactive decay; only the number of neutrons alters.
Conclusion: The Significance of 17 Protons
The seemingly simple question, "How many protons does chloride have?", leads to a deeper understanding of atomic structure, chemical behavior, and isotopic variations. The 17 protons in a chloride ion are the defining characteristic of chlorine, dictating its place on the periodic table, its electron configuration, its reactivity, and its role in forming ionic compounds. Understanding this fundamental aspect of atomic structure is essential for comprehending the vast and complex world of chemistry and its applications in countless areas of science and technology. The consistent presence of 17 protons across all chlorine isotopes highlights the fundamental nature of atomic number as the defining characteristic of an element. This consistent proton count underlies all the chemical and physical properties associated with this crucial element.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Chloride Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
