How Many Neutrons Does Mg Have
News Leon
Mar 19, 2025 · 5 min read
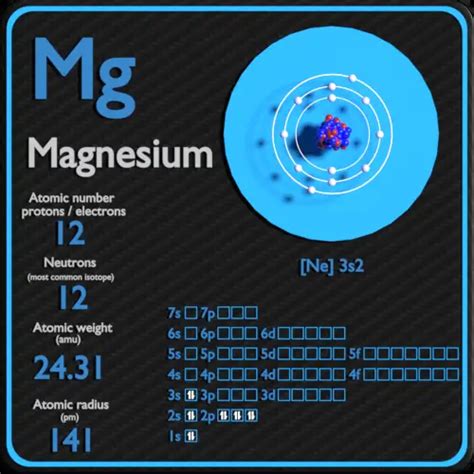
Table of Contents
How Many Neutrons Does Mg Have? A Deep Dive into Magnesium Isotopes
Magnesium (Mg), the element with atomic number 12, is a vital element for life, playing a crucial role in numerous biological processes. Understanding its atomic structure, particularly the number of neutrons in its nucleus, is key to grasping its properties and behavior. But the answer isn't a simple single number. The number of neutrons in magnesium varies depending on the isotope of magnesium in question. This article explores the different isotopes of magnesium, their neutron counts, abundance in nature, and their applications.
Understanding Isotopes and Atomic Structure
Before delving into the specifics of magnesium isotopes, let's briefly review some fundamental concepts:
-
Atomic Number: This represents the number of protons in an atom's nucleus. For magnesium, the atomic number is always 12. This defines the element as magnesium.
-
Mass Number: This is the total number of protons and neutrons in the nucleus. It's an approximation of the atomic mass.
-
Isotopes: Atoms of the same element (same atomic number) but with different numbers of neutrons (and therefore different mass numbers) are called isotopes. These isotopes exhibit similar chemical properties but may differ slightly in physical properties.
-
Neutron: A subatomic particle residing in the nucleus, carrying no net electric charge and possessing a mass slightly larger than that of a proton.
Magnesium Isotopes and Their Neutron Counts
Magnesium has three naturally occurring stable isotopes:
Magnesium-24 (²⁴Mg)
- Abundance: Approximately 79% of naturally occurring magnesium is ²⁴Mg.
- Protons: 12
- Neutrons: 24 (mass number) - 12 (protons) = 12
- Applications: Like other magnesium isotopes, ²⁴Mg finds use in various applications, its abundance making it readily available for different industrial processes.
Magnesium-25 (²⁵Mg)
- Abundance: Approximately 10% of naturally occurring magnesium is ²⁵Mg.
- Protons: 12
- Neutrons: 25 (mass number) - 12 (protons) = 13
- Applications: Its use is often intertwined with other magnesium isotopes in industrial and research settings. The slight mass difference compared to ²⁴Mg can be significant in certain spectroscopic techniques.
Magnesium-26 (²⁶Mg)
- Abundance: Approximately 11% of naturally occurring magnesium is ²⁶Mg.
- Protons: 12
- Neutrons: 26 (mass number) - 12 (protons) = 14
- Applications: Similar to ²⁵Mg, its applications often overlap with other magnesium isotopes. The slightly higher neutron count can have subtle effects in specific applications, such as nuclear magnetic resonance (NMR) spectroscopy.
Radioactive Isotopes of Magnesium
Besides the three stable isotopes, several radioactive isotopes of magnesium exist, although they are not naturally found in significant quantities on Earth. These are produced artificially through nuclear reactions. These radioactive isotopes have varying half-lives and decay modes, making them useful in specific scientific and medical applications. Examples include:
- ²⁷Mg: This radioactive isotope decays through beta emission.
- ²⁸Mg: Also undergoes beta decay.
- Other heavier radioactive magnesium isotopes exist but have extremely short half-lives.
The number of neutrons in these radioactive isotopes is calculated the same way as the stable isotopes: mass number minus the atomic number (12).
The Significance of Isotopic Ratios
The relative abundances of magnesium isotopes (²⁴Mg, ²⁵Mg, and ²⁶Mg) are not uniform across all geological samples. Variations in these isotopic ratios are used in several scientific disciplines, including:
-
Geochemistry: Isotope ratios can provide insights into geological processes, such as the formation of rocks and minerals, and the evolution of the Earth's mantle. Differences in isotopic ratios can reveal the origin and mixing of different geological materials.
-
Cosmochemistry: The isotopic composition of magnesium in meteorites provides clues to the formation of the solar system and the processes that occurred in the early solar nebula. Comparing isotopic ratios in terrestrial and extraterrestrial samples helps scientists understand the history of our solar system.
-
Paleoclimatology: Variations in magnesium isotope ratios in ocean sediments can be used as proxies for past climate change, reflecting changes in ocean temperature and salinity.
-
Environmental Science: Isotopic tracing techniques employing magnesium isotopes can be valuable tools in monitoring environmental processes and pollution.
Applications of Magnesium and its Isotopes
Magnesium, due to its properties and abundance, finds extensive applications in various industries:
-
Materials Science: Magnesium alloys are lightweight and strong, making them valuable in the aerospace and automotive industries. The specific properties of the alloys are influenced by the isotopic composition.
-
Biomedical Applications: Magnesium plays a critical role in human biology, acting as a cofactor in numerous enzymatic reactions. Magnesium salts and compounds find applications in various pharmaceutical preparations. Radioactive isotopes can be used in medical imaging and treatments.
-
Chemical Industry: Magnesium is used as a reducing agent in the production of certain metals and as a component in various chemical compounds.
-
Pyrotechnics: Magnesium burns brightly and is commonly used in fireworks and flares.
Conclusion: The Importance of Precision
While a simple answer to "how many neutrons does Mg have?" might seem to be "around 12", a deeper understanding reveals a more nuanced truth. The number of neutrons in magnesium varies, depending on the specific isotope. The three naturally occurring stable isotopes, ²⁴Mg, ²⁵Mg, and ²⁶Mg, each possess a different number of neutrons (12, 13, and 14, respectively). Furthermore, the relative abundance and isotopic ratios of these isotopes hold significant value in various scientific fields. Understanding this complexity is crucial for researchers and scientists working with magnesium in different contexts. From materials science to geological dating and biomedical applications, the precise number of neutrons in a magnesium atom can have profound implications on its properties and applications. The variations in isotopic ratios offer powerful tools for investigating a wide array of scientific questions, highlighting the importance of going beyond simplistic answers and embracing the intricacies of isotopic chemistry.
Latest Posts
Latest Posts
-
Identify The Two Key Factors That Determine Nuclear Stability
Mar 19, 2025
-
What Is 2 5 As A Percent
Mar 19, 2025
-
Write The Formula For Sulfurous Acid
Mar 19, 2025
-
How Is A Prokaryotic And Eukaryotic Cell Similar
Mar 19, 2025
-
The Urinary Bladder And Ureters Are Lined By
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Mg Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
