How Many Neutrons Does Gold Have
News Leon
Mar 17, 2025 · 5 min read
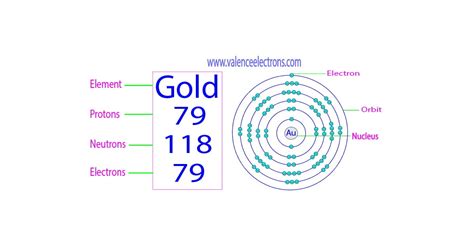
Table of Contents
How Many Neutrons Does Gold Have? Unraveling the Atomic Structure of Gold
Gold, a lustrous, malleable, and ductile metal known for its beauty and value, has captivated humanity for millennia. But beyond its aesthetic appeal and economic significance lies a fascinating world of atomic structure. One key aspect of understanding gold's properties is determining the number of neutrons in its nucleus. This seemingly simple question opens a door to a deeper exploration of isotopes, atomic mass, and the intricacies of nuclear physics.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the neutron count of gold, let's establish a fundamental understanding of atomic structure. Every atom consists of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; gold, for example, always has 79 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the concept of isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom.
Isotopes: The Key to Variable Neutron Numbers
The term "isotope" refers to atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. Gold, like all elements, has several isotopes, each with a slightly different mass due to varying neutron counts.
Gold's Most Abundant Isotope: ¹⁹⁷Au
The most prevalent isotope of gold is ¹⁹⁷Au. The "197" represents the mass number, which is the sum of protons and neutrons. Since gold always has 79 protons, the number of neutrons in ¹⁹⁷Au can be calculated:
197 (mass number) - 79 (protons) = 118 neutrons
Therefore, the most common form of gold atoms contains 118 neutrons.
Other Gold Isotopes and Their Neutron Counts
While ¹⁹⁷Au is the most abundant gold isotope, several other isotopes exist, albeit in much smaller quantities. These isotopes are radioactive, meaning they decay over time, transforming into different elements. Some examples of other gold isotopes and their neutron counts include:
- ¹⁹⁵Au: This isotope has a mass number of 195 and therefore contains 195 - 79 = 116 neutrons.
- ¹⁹⁸Au: With a mass number of 198, this isotope possesses 198 - 79 = 119 neutrons.
- ¹⁹⁹Au: This isotope has 199 - 79 = 120 neutrons.
These variations in neutron number affect the stability and radioactivity of the gold isotopes. The more neutron-rich or neutron-poor isotopes are generally less stable and undergo radioactive decay.
The Significance of Neutron Number in Gold's Properties
The number of neutrons in a gold atom subtly influences its properties. While the chemical behavior remains largely consistent across isotopes (due to the unchanging number of protons), the differing mass affects physical properties like density and reactivity in some nuclear processes. For instance, the slight mass differences between gold isotopes can be exploited in certain scientific techniques like isotopic analysis, which allows researchers to trace the origins and movement of gold through various systems.
Calculating Neutron Numbers in Other Elements
The method used to calculate the number of neutrons in gold atoms can be applied to any element. You simply need to know the element's atomic number (number of protons) and the mass number of the specific isotope in question. The formula remains consistent:
Number of neutrons = Mass number - Atomic number
This fundamental principle is crucial in understanding nuclear chemistry, radioactivity, and the behavior of various elements.
Applications of Isotopic Analysis and Gold's Neutron Composition
The varying neutron compositions in gold isotopes have practical applications in various fields:
- Archaeology: Isotopic analysis of gold artifacts can help determine the source of the gold, providing valuable insights into ancient trade routes and metallurgical practices.
- Geology: Gold isotopes are used to study geological processes and the formation of ore deposits.
- Medicine: Radioactive gold isotopes like ¹⁹⁸Au are sometimes employed in nuclear medicine for therapeutic purposes, though other elements are now more commonly used.
- Nuclear Physics: Studying the nuclear structure and decay of gold isotopes contributes to our fundamental understanding of nuclear physics and radioactivity.
Further Exploration: Nuclear Stability and Binding Energy
The number of neutrons in an atom is directly related to its nuclear stability. The ratio of protons to neutrons plays a crucial role in determining whether an isotope will be stable or radioactive. For lighter elements, a roughly equal number of protons and neutrons tends to lead to stability. However, for heavier elements like gold, a higher neutron-to-proton ratio is needed to achieve stability. This is because the strong nuclear force, which holds the nucleus together, has a shorter range than the electromagnetic force (repulsion between positively charged protons). More neutrons help to counteract the repulsive forces between protons and maintain the integrity of the nucleus. The concept of binding energy, the energy required to disassemble a nucleus into its constituent protons and neutrons, is directly related to nuclear stability and isotope characteristics. Elements with higher binding energy per nucleon are generally more stable.
Conclusion: The multifaceted nature of Gold's Neutron Count
The seemingly simple question of "how many neutrons does gold have?" reveals the intricate and fascinating world of atomic structure, isotopes, and nuclear physics. While the most abundant gold isotope, ¹⁹⁷Au, contains 118 neutrons, other isotopes exist with varying neutron numbers, affecting their stability and applications in various fields. Understanding the relationship between proton and neutron numbers is crucial for comprehending the properties and behavior of elements, their isotopes, and their significance in diverse scientific and technological applications. The study of gold's isotopic composition continues to provide valuable insights into the fundamental laws governing the universe and their implications for our world. From ancient artifacts to modern scientific research, the story of gold's neutrons is far from over. Further exploration into nuclear physics and isotopic analysis promises to uncover even more fascinating secrets of this precious metal and the atomic world it represents.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Gold Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
