How Many Electrons Does Chloride Have
News Leon
Mar 24, 2025 · 6 min read
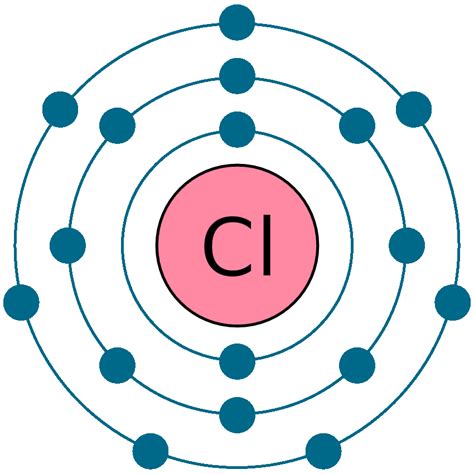
Table of Contents
How Many Electrons Does Chloride Have? A Deep Dive into Atomic Structure and Ion Formation
Understanding the number of electrons in an atom or ion is fundamental to comprehending chemistry. This article will delve into the specifics of chloride, exploring its electron configuration, the process of ion formation, and its implications in various chemical contexts. We'll also touch upon related concepts to provide a comprehensive understanding of this important chemical species.
The Neutral Chlorine Atom: Starting Point
Before we examine chloride, let's understand its parent atom: chlorine. Chlorine (Cl) is a chemical element with an atomic number of 17. This atomic number signifies that a neutral chlorine atom possesses 17 protons in its nucleus. Crucially, in a neutral atom, the number of protons always equals the number of electrons. Therefore, a neutral chlorine atom has 17 electrons.
Electron Configuration: Distributing the Electrons
These 17 electrons are not randomly distributed around the nucleus. They occupy specific energy levels or shells, following the principles of quantum mechanics. The electron configuration of chlorine is: 1s²2s²2p⁶3s²3p⁵.
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s orbital.
- 2s²: Two electrons in the second energy level (n=2), in the s orbital.
- 2p⁶: Six electrons in the second energy level (n=2), in the three p orbitals (px, py, pz). Each p orbital can hold a maximum of two electrons.
- 3s²: Two electrons in the third energy level (n=3), in the s orbital.
- 3p⁵: Five electrons in the third energy level (n=3), in the three p orbitals.
This electron configuration explains chlorine's chemical reactivity. The outermost shell (the valence shell – n=3 in this case) contains seven electrons. Atoms strive for stability, often achieved by having a full outermost shell, typically containing eight electrons (the octet rule). This drive for stability dictates chlorine's behavior in chemical reactions.
The Chloride Ion: Gaining an Electron
Chloride (Cl⁻) is not a neutral chlorine atom; it's an anion, a negatively charged ion. It forms when a chlorine atom gains one electron. This electron addition completes the outermost shell, creating a stable electron configuration identical to that of argon (Ar), a noble gas with a full octet.
Therefore, a chloride ion (Cl⁻) has 18 electrons. This is because it has gained one electron to the neutral chlorine atom's 17 electrons. The addition of a negatively charged electron results in a net negative charge on the ion.
The Role of Electronegativity
Chlorine's high electronegativity plays a crucial role in its ability to gain an electron. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Chlorine is highly electronegative, meaning it strongly attracts electrons. This attraction is what enables it to readily gain an electron from another atom, forming the chloride ion.
Chemical Reactions and Chloride Formation
Chloride ions are ubiquitous in various chemical contexts. They commonly form through ionic bonding, where a chlorine atom accepts an electron from a less electronegative atom, typically a metal. Consider the formation of sodium chloride (NaCl), common table salt:
- Sodium (Na) has one electron in its outermost shell and readily loses it to achieve a stable octet.
- Chlorine (Cl) readily accepts this electron to complete its own octet.
This electron transfer results in the formation of Na⁺ (sodium cation) and Cl⁻ (chloride anion), held together by electrostatic attraction (ionic bond) forming the crystalline structure of NaCl.
Many other compounds contain chloride ions, including:
- Hydrochloric acid (HCl): A strong acid found in the stomach, where it plays a vital role in digestion.
- Calcium chloride (CaCl₂): Used as a de-icer and in various industrial applications.
- Magnesium chloride (MgCl₂): Used in various industrial processes, including the production of magnesium metal.
- Potassium chloride (KCl): Used in fertilizers and as a salt substitute.
These are just a few examples of the countless compounds containing chloride ions. The chloride ion's stability and reactivity make it an essential component in a wide range of chemical systems.
Implications in Biology and Medicine
Chloride ions are not only important in inorganic chemistry; they also play crucial roles in biological systems. They are one of the major anions in extracellular fluid, contributing significantly to maintaining osmotic balance and electrolyte balance within the body. This balance is critical for proper cellular function and overall health.
Chloride channels, specialized protein structures within cell membranes, regulate the movement of chloride ions across cell membranes. These channels are involved in various physiological processes, including nerve impulse transmission, muscle contraction, and fluid secretion. Dysfunctions in chloride channels are associated with several diseases, highlighting the importance of proper chloride ion regulation.
Chloride Ion in Analytical Chemistry
The abundance and reactivity of chloride ions make them easily detectable using various analytical techniques. These techniques include:
- Titration: Using silver nitrate (AgNO₃) to precipitate chloride ions as silver chloride (AgCl), a white precipitate. This allows for the quantitative determination of chloride concentration in a sample.
- Spectroscopy: Different spectroscopic methods can be employed to detect and quantify chloride ions, based on their interactions with electromagnetic radiation.
- Chromatography: Techniques such as ion chromatography can separate and quantify chloride ions from a mixture of other ions.
These methods are used extensively in environmental monitoring, food analysis, and clinical diagnostics, among other applications.
Beyond the Basics: Isotopes and Abundance
Chlorine has two main isotopes: ³⁵Cl and ³⁷Cl. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Both isotopes have 17 protons and 17 electrons when neutral, but they differ in the number of neutrons (18 for ³⁵Cl and 20 for ³⁷Cl). This difference in neutron number doesn't affect the electron count, but it does impact the atom's mass and stability. The relative abundance of these isotopes impacts the average atomic mass of chlorine, which is approximately 35.45 amu.
Conclusion
In conclusion, a chloride ion (Cl⁻) possesses 18 electrons. This extra electron, gained from a neutral chlorine atom, results in a stable electron configuration, crucial for its extensive involvement in chemical reactions and biological processes. Understanding the electron configuration and ion formation of chloride provides a foundation for grasping various chemical concepts and the crucial roles played by chloride ions in both natural and man-made systems. From its presence in table salt to its vital roles in biological systems and its detection in various analytical methods, the chloride ion remains a central and widely studied chemical species. The simple answer – 18 electrons – belies the richness and complexity of this fundamental chemical component.
Latest Posts
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Chloride Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.