How Many Electrons Are In 1 Coulomb
News Leon
Mar 16, 2025 · 5 min read
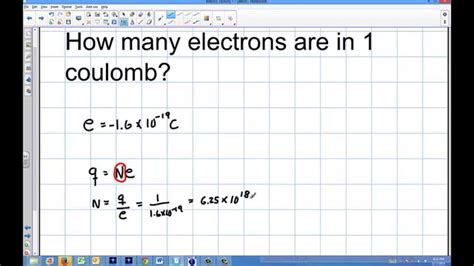
Table of Contents
How Many Electrons are in 1 Coulomb? Unpacking the Fundamental Charge
The seemingly simple question, "How many electrons are in 1 coulomb?" delves into the heart of electromagnetism and fundamental physics. Understanding this requires a grasp of key concepts like electric charge, Coulomb's Law, and Avogadro's number. This article will provide a comprehensive explanation, exploring the calculations and underlying principles involved. We'll also touch upon the historical context and practical applications of this fundamental relationship.
Understanding the Coulomb and Elementary Charge
Before diving into the calculation, let's define our terms. A coulomb (C) is the International System of Units (SI) unit of electric charge. It represents a significant amount of charge – a static discharge from your body might be on the order of a few nanocoulombs (nC), or billionths of a coulomb.
The elementary charge (e) is the fundamental unit of electric charge. It's the magnitude of the charge carried by a single proton or, conversely, the magnitude of the charge of a single electron. This charge is incredibly small: approximately 1.602 x 10<sup>-19</sup> Coulombs. This means that a coulomb represents a massive number of elementary charges.
Calculating the Number of Electrons in 1 Coulomb
To determine the number of electrons in 1 coulomb, we can use a simple yet powerful calculation:
Number of electrons = Total charge (in Coulombs) / Elementary charge (in Coulombs)
Plugging in the values:
Number of electrons = 1 C / (1.602 x 10<sup>-19</sup> C/electron)
This calculation reveals that there are approximately 6.24 x 10<sup>18</sup> electrons in 1 coulomb of charge. This is a staggering number, highlighting just how incredibly small the elementary charge is.
The Significance of Avogadro's Number
While the above calculation is straightforward, it's worth considering the connection to Avogadro's number. Avogadro's number (approximately 6.022 x 10<sup>23</sup>) represents the number of constituent particles (atoms, molecules, ions, etc.) in one mole of a substance.
While not directly used in the calculation above, Avogadro's number plays a crucial role in relating macroscopic quantities (like moles) to microscopic quantities (like the number of electrons). For instance, if you're working with a problem involving the charge of a mole of electrons, Avogadro's number would be essential in the calculation.
Historical Context: The Development of the Coulomb and Elementary Charge
The concept of electric charge and its measurement have a rich history. The coulomb is named after Charles-Augustin de Coulomb, a French physicist who in the late 18th century established Coulomb's Law, which describes the force between two charged objects. His meticulous experiments helped solidify our understanding of electrostatic forces.
The determination of the elementary charge, however, was a much later achievement. Many scientists contributed to its measurement, with Robert Millikan's oil-drop experiment in the early 20th century being a landmark achievement. Millikan's experiment provided a precise measurement of the elementary charge, confirming its discrete nature and providing crucial experimental validation for the atomic theory of matter.
Practical Applications and Real-World Examples
The relationship between coulombs and electrons has numerous practical applications across various fields:
1. Electronics and Electrical Engineering:
Understanding the flow of electrons (electric current) is fundamental to electronics. Current, measured in amperes (A), is defined as the rate of flow of charge (coulombs per second). Thus, the number of electrons passing a point in a circuit per second is directly related to the current.
2. Electrochemistry:
Electrochemistry involves chemical reactions driven by electric current or producing electric current. Faraday's laws of electrolysis relate the amount of substance deposited or liberated at an electrode to the quantity of electric charge passed through the cell. This directly involves the number of electrons involved in the redox reactions.
3. Particle Physics:
In particle physics, understanding elementary charges is crucial for analyzing particle interactions and decays. The charge of particles plays a central role in determining their behavior in electromagnetic fields and their interactions with other particles.
4. Semiconductor Physics:
The behavior of semiconductors is intrinsically linked to the movement of electrons and holes (electron vacancies). Doping semiconductors involves introducing impurities to control the number of charge carriers, influencing the electrical conductivity. Understanding the relationship between coulombs and electrons is crucial for designing and optimizing semiconductor devices.
Beyond the Basics: Considering the Context
While the calculation of 6.24 x 10<sup>18</sup> electrons in a coulomb is a fundamental result, it's important to consider the context:
-
Free vs. Bound Electrons: The calculation assumes that all electrons are free to move and contribute to the total charge. In reality, some electrons are tightly bound to atoms and don't contribute to bulk charge movement.
-
Charge Carriers: In certain materials (like semiconductors), charge transport can involve both electrons and "holes" (the absence of an electron, which acts like a positive charge carrier). The total charge would then be a combination of electrons and holes.
-
Quantum Effects: At the nanoscale, quantum mechanical effects become important, and the simple classical model of charge might not fully describe the behavior of electrons.
Conclusion: A Fundamental Relationship with Far-Reaching Consequences
The seemingly simple question of how many electrons are in a coulomb leads to a deeper understanding of fundamental physics and the nature of electric charge. The calculation, while straightforward, reveals the immense number of electrons comprising even a small amount of charge. This fundamental relationship underpins our understanding of electronics, electrochemistry, particle physics, and many other scientific and technological disciplines. The continuing exploration of the interactions and behavior of these fundamental particles remains at the forefront of scientific research, pushing the boundaries of our knowledge and leading to technological innovations. The seemingly simple calculation provides a powerful foundation for a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In 1 Coulomb . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
