How Many Atoms In A Bcc Unit Cell
News Leon
Apr 06, 2025 · 5 min read
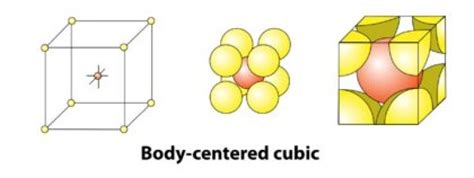
Table of Contents
How Many Atoms are in a BCC Unit Cell? A Deep Dive into Body-Centered Cubic Structures
Understanding crystal structures is fundamental in materials science and solid-state physics. One of the most common crystal structures is the body-centered cubic (BCC) structure. A frequent question that arises is: how many atoms are actually in a BCC unit cell? This article will delve deep into this question, explaining not only the answer but also the underlying concepts and implications.
Understanding Unit Cells: The Building Blocks of Crystals
Before tackling the BCC unit cell specifically, let's establish a foundational understanding of unit cells. A unit cell is the smallest repeating unit in a crystal lattice. Think of it as the fundamental building block that, when repeated in three dimensions, creates the entire crystal structure. There are several types of unit cells, including simple cubic, body-centered cubic (BCC), and face-centered cubic (FCC). The arrangement of atoms within the unit cell determines the overall crystal structure and its properties.
Visualizing the Unit Cell: A Three-Dimensional Puzzle
Imagine a cube. This cube represents our unit cell. The atoms within this cube are positioned in specific locations, and their arrangement dictates the type of unit cell. The positions of these atoms define the crystal lattice. The crystal lattice is an abstract framework that shows the arrangement of atoms or ions in a crystalline material. It’s important to note that the atoms themselves occupy space and are not merely points in this abstract framework.
Decoding the BCC Unit Cell: Atoms at the Center
The body-centered cubic (BCC) structure is named for its characteristic arrangement: one atom resides at each of the eight corners of the cube, and one additional atom is located precisely at the center of the cube. This central atom is crucial in understanding the total number of atoms within the unit cell.
Corner Atoms: Shared Ownership
It's crucial to understand that the corner atoms are shared between adjacent unit cells. Each corner atom is shared by eight adjacent unit cells. Therefore, each corner atom only contributes 1/8 of an atom to a single unit cell. Since there are eight corners, the contribution from the corner atoms to a single unit cell is (1/8) * 8 = 1 atom.
The Central Atom: Sole Possession
Unlike the corner atoms, the atom at the center of the BCC unit cell belongs entirely to that specific unit cell. It is not shared with any neighboring unit cells. This gives a full contribution of 1 atom.
Calculating the Total Number of Atoms: The Final Summation
Now we can calculate the total number of atoms within a single BCC unit cell:
- Corner atoms: 8 corner atoms * (1/8 atom/corner) = 1 atom
- Center atom: 1 atom
Total atoms in a BCC unit cell: 1 + 1 = 2 atoms
Therefore, there are two atoms in a BCC unit cell. This seemingly simple answer highlights a crucial concept in crystallography: the importance of understanding atom sharing between unit cells.
BCC Structure: Properties and Examples
The BCC structure has distinct properties due to its atomic arrangement. These properties influence the materials that adopt this structure.
Properties stemming from BCC structure:
- High packing efficiency: Although less densely packed than FCC, BCC structures still have a reasonably high atomic packing factor (APF). The APF represents the fraction of volume in a unit cell that is occupied by atoms. For BCC, it's approximately 68%, allowing for relatively strong metallic bonding.
- Ductility and malleability: The specific slip systems in BCC crystals allow for some degree of deformation before fracture, leading to moderate ductility and malleability. However, this can be temperature-dependent.
- High strength: The arrangement of atoms in the BCC structure leads to a higher degree of resistance to deformation at lower temperatures, resulting in a higher strength compared to some other structures.
- Anisotropy: The BCC structure displays anisotropy, meaning its properties can vary depending on the crystallographic direction. This is because the atomic arrangement isn't uniform in all directions.
Examples of BCC Metals:
Many metals and alloys exhibit a BCC structure at certain temperatures. Some common examples include:
- Iron (α-iron): Iron adopts a BCC structure at room temperature. This is a crucial factor in its properties and applications.
- Chromium: This transition metal is a common example of a BCC metal known for its high corrosion resistance.
- Tungsten: Known for its high melting point, tungsten also adopts a BCC structure.
- Molybdenum: Another high-melting-point metal with a BCC structure used in high-temperature applications.
- Vanadium: A transition metal with a BCC structure used in certain alloys.
Beyond the Basics: Applications and Further Considerations
The BCC structure's properties have numerous applications in various fields:
- Steel Manufacturing: The BCC structure of iron at room temperature plays a significant role in the properties of steel. Alloying elements modify the properties of iron, leading to a wide range of steel types with different strengths, ductilities, and other characteristics.
- High-Temperature Applications: Metals like tungsten and molybdenum, with their high melting points and BCC structures, are utilized in applications that require high-temperature resistance, such as incandescent light bulbs and rocket nozzles.
- Nuclear Reactors: Certain BCC metals have properties that make them suitable for components in nuclear reactors, where resistance to radiation damage and high temperatures are essential.
Factors influencing BCC structure stability:
Several factors influence whether a metal will adopt a BCC structure:
- Atomic size and radius: The relative size of atoms influences the way they pack together, affecting whether a BCC structure is energetically favorable.
- Electron configuration: The number and arrangement of electrons in the outermost shell of an atom can significantly influence the bonding and stability of different crystal structures.
- Temperature: Temperature changes can induce phase transitions, causing a metal to switch from one crystal structure (like BCC) to another.
Conclusion: A Comprehensive Understanding of BCC Unit Cells
Determining the number of atoms in a BCC unit cell – a seemingly simple calculation – underscores the fundamental principles of crystallography. Understanding the concept of atom sharing between unit cells is essential for accurately determining the atomic composition within any crystal structure. This fundamental knowledge forms the basis for understanding the properties and applications of countless materials in various fields of engineering and technology. The BCC structure, with its two atoms per unit cell and unique properties, is a crucial example of how crystal structure significantly impacts material behavior. Further investigation into the complexities of crystal structures offers an exciting path for innovation and discovery.
Latest Posts
Latest Posts
-
What Is The Largest Phylum Of Invertebrates
Apr 08, 2025
-
Do Cheek Cells Have A Nucleus
Apr 08, 2025
-
What Is 0 8 Repeating As A Fraction
Apr 08, 2025
-
The Smallest Contractile Unit Of Muscle Is A
Apr 08, 2025
-
Which Statement About Fusion Is Correct
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms In A Bcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.