How Many Atoms In 0.075 Mol Of Titanium
News Leon
Apr 03, 2025 · 5 min read
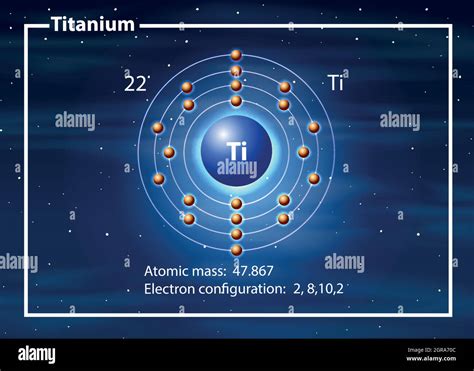
Table of Contents
- How Many Atoms In 0.075 Mol Of Titanium
- Table of Contents
- How Many Atoms Are in 0.075 Mol of Titanium? A Deep Dive into Moles, Atoms, and Avogadro's Number
- Understanding Moles and Avogadro's Number
- Calculating Atoms in 0.075 Mol of Titanium
- Significance of Molar Mass in Related Calculations
- Applications in Different Scientific Fields
- 1. Material Science and Engineering:
- 2. Chemistry and Biochemistry:
- 3. Nuclear Physics and Radioactivity:
- 4. Environmental Science:
- 5. Astrophysics and Cosmology:
- Beyond Titanium: Extending the Concept
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How Many Atoms Are in 0.075 Mol of Titanium? A Deep Dive into Moles, Atoms, and Avogadro's Number
Determining the number of atoms in a given amount of substance is a fundamental concept in chemistry. This article will walk you through the calculation of atoms in 0.075 mol of titanium, explaining the underlying principles and offering insights into related concepts like Avogadro's number and molar mass. We'll also explore the broader significance of these calculations in various scientific fields.
Understanding Moles and Avogadro's Number
Before tackling the calculation, let's establish a solid understanding of the key concepts involved:
Moles: A mole (mol) is a fundamental unit in chemistry that represents a specific number of particles, be it atoms, molecules, ions, or other entities. It's a way to relate the microscopic world of atoms and molecules to the macroscopic world we observe. One mole of any substance contains the same number of particles.
Avogadro's Number: This crucial constant, approximately 6.022 x 10<sup>23</sup>, represents the number of particles (atoms, molecules, etc.) in one mole of a substance. This vast number highlights the incredibly small scale of atoms and molecules. It's named after Amedeo Avogadro, an Italian scientist whose work laid the foundation for this concept. Avogadro's number is essential for converting between moles and the number of atoms or molecules.
Calculating Atoms in 0.075 Mol of Titanium
Now, let's apply these concepts to calculate the number of atoms in 0.075 mol of titanium (Ti). The calculation is straightforward:
-
Start with the given amount of substance: We have 0.075 mol of titanium.
-
Use Avogadro's number: We know that 1 mol of any substance contains 6.022 x 10<sup>23</sup> particles.
-
Set up the conversion factor: We can set up a conversion factor using Avogadro's number to convert from moles to atoms:
(6.022 x 10<sup>23</sup> atoms Ti / 1 mol Ti)
-
Perform the calculation: Multiply the given moles of titanium by the conversion factor:
0.075 mol Ti x (6.022 x 10<sup>23</sup> atoms Ti / 1 mol Ti) = 4.5165 x 10<sup>22</sup> atoms Ti
Therefore, there are approximately 4.5165 x 10<sup>22</sup> atoms in 0.075 mol of titanium.
Significance of Molar Mass in Related Calculations
While this calculation focused on the number of atoms, it's important to understand the role of molar mass. Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). For titanium, the molar mass is approximately 47.87 g/mol.
This allows for conversions between mass, moles, and the number of atoms. For example, if we were given the mass of titanium instead of the number of moles, we could first convert the mass to moles using the molar mass, and then use Avogadro's number to find the number of atoms. The relationship can be summarized as follows:
- Mass (g) / Molar Mass (g/mol) = Moles (mol)
- Moles (mol) x Avogadro's Number (atoms/mol) = Number of Atoms
This interplay between mass, moles, and the number of atoms is crucial in various chemical calculations and analyses.
Applications in Different Scientific Fields
The ability to determine the number of atoms in a given amount of substance has wide-ranging applications across numerous scientific fields:
1. Material Science and Engineering:
Understanding the number of atoms is crucial in material science for designing and characterizing new materials. The atomic structure and arrangement directly influence a material's properties, such as strength, conductivity, and reactivity. Calculations involving Avogadro's number help engineers determine the optimal composition and structure for materials used in various applications, from aerospace engineering to biomedical implants.
2. Chemistry and Biochemistry:
In chemistry and biochemistry, precise calculations involving moles and Avogadro's number are fundamental for stoichiometry – the study of the quantitative relationships between reactants and products in chemical reactions. These calculations allow chemists to predict the amount of product formed from a given amount of reactant, optimize reaction conditions, and analyze reaction yields. They are vital in areas such as synthesis, analysis, and drug development.
3. Nuclear Physics and Radioactivity:
In nuclear physics, understanding the number of atoms is essential for calculations related to radioactivity and nuclear reactions. The decay of radioactive isotopes is governed by the number of radioactive atoms present, and calculations involving Avogadro's number are used to predict the rate of decay and the half-life of radioactive materials. This is vital in applications such as radioisotope dating, nuclear medicine, and radiation safety.
4. Environmental Science:
Environmental scientists use these calculations to analyze pollutant concentrations and assess environmental impact. Determining the number of pollutant molecules or atoms in a given volume of air or water helps researchers understand the extent of pollution and its effects on ecosystems. This information is crucial for developing effective environmental policies and remediation strategies.
5. Astrophysics and Cosmology:
While seemingly far removed, the principles of moles and Avogadro's number also play a role in astrophysics and cosmology. Scientists use these concepts to estimate the abundance of elements in stars and galaxies, which provides crucial insights into the processes that govern stellar evolution and the formation of the universe.
Beyond Titanium: Extending the Concept
The principles discussed here apply to any element or compound. To calculate the number of atoms in a given amount of any substance, simply replace the molar mass and Avogadro's number with the appropriate values for that specific substance. The fundamental relationship between moles, Avogadro's number, and the number of atoms remains consistent.
Conclusion
Calculating the number of atoms in 0.075 mol of titanium, or any other substance, involves a simple yet powerful application of Avogadro's number and the concept of moles. This calculation, while seemingly basic, underpins a vast array of scientific disciplines and has significant practical applications in various fields. Understanding the connection between moles, Avogadro's number, and the number of atoms is essential for anyone pursuing studies or careers in science, engineering, and related fields. The ability to perform these calculations accurately is a foundational skill for success in these areas. Moreover, appreciating the magnitude of Avogadro's number helps us grasp the scale of the microscopic world and its impact on our macroscopic reality.
Latest Posts
Latest Posts
-
Select The Correct Mechanism Of Stomatal Opening And Closing
Apr 09, 2025
-
What Is The Relationship Between The Following Two Molecules
Apr 09, 2025
-
What Is The Transcription Product Of The Sequence Gctagcgatgac
Apr 09, 2025
-
Find Three Consecutive Integers Whose Sum Is 36
Apr 09, 2025
-
What Is The Molar Mass Of Silver Nitrate
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms In 0.075 Mol Of Titanium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.