How Many Atoms Are In A Bcc Unit Cell
News Leon
Mar 14, 2025 · 7 min read
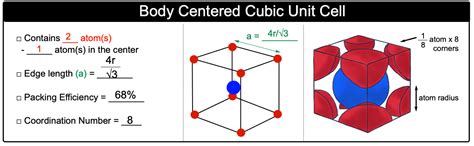
Table of Contents
How Many Atoms Are in a BCC Unit Cell? A Deep Dive into Body-Centered Cubic Structures
Understanding crystal structures is fundamental to materials science and many other scientific disciplines. One common crystal structure is the body-centered cubic (BCC) structure. A crucial aspect of understanding BCC structures is determining the number of atoms within a single unit cell. This article will delve into the intricacies of BCC unit cells, providing a detailed explanation of atom count, coordination number, and packing efficiency, along with relevant calculations and illustrations.
Understanding Unit Cells and Crystal Structures
Before we dive into the specifics of the BCC unit cell, let's establish a foundational understanding of crystal structures and unit cells. Crystals are characterized by their highly ordered, repeating arrangements of atoms, ions, or molecules. This repeating pattern is represented by a unit cell, which is the smallest repeating unit that, when stacked in three dimensions, creates the entire crystal lattice. Different types of unit cells exist, including simple cubic (SC), body-centered cubic (BCC), and face-centered cubic (FCC).
The Body-Centered Cubic (BCC) Structure: A Visual Representation
The BCC structure gets its name from the arrangement of atoms within its unit cell. Imagine a cube. In a BCC unit cell, there is one atom at each of the eight corners of the cube, and one atom positioned exactly in the center of the cube. This central atom is entirely within the unit cell, unlike the corner atoms which are shared between adjacent unit cells.
This arrangement leads to a unique set of properties and characteristics for materials exhibiting a BCC structure. Understanding this arrangement is key to calculating the number of atoms per unit cell.
Visualizing Atom Contribution in a BCC Unit Cell
Each of the eight corner atoms contributes 1/8th of its volume to the unit cell. This is because each corner atom is shared equally among eight adjacent unit cells. Therefore, the contribution from the corner atoms is (8 corner atoms) * (1/8 atom/corner atom) = 1 atom.
The centrally located atom is completely contained within the unit cell, contributing a full atom.
Therefore, the total number of atoms in a BCC unit cell is:
1 (from corner atoms) + 1 (from the central atom) = 2 atoms
Calculating Atomic Packing Factor (APF) for a BCC Unit Cell
The Atomic Packing Factor (APF) represents the fraction of volume in a unit cell that is occupied by atoms. It is a crucial parameter for understanding the density and other physical properties of materials. For a BCC unit cell, the APF calculation involves determining the volume occupied by atoms and dividing it by the total volume of the unit cell.
Steps to Calculate APF for BCC
-
Volume of atoms: Assuming atoms are hard spheres, the volume of a single atom is (4/3)πr³, where 'r' is the atomic radius. Since a BCC unit cell contains two atoms, the total volume occupied by atoms is 2 * (4/3)πr³.
-
Relationship between atomic radius and unit cell edge length: In a BCC unit cell, the body diagonal can be expressed as 4r (four times the atomic radius). Using the Pythagorean theorem in three dimensions, we can relate the body diagonal (4r) to the unit cell edge length (a): (4r)² = a² + a² + a² = 3a². Solving for 'a', we get a = 4r/√3.
-
Volume of the unit cell: The volume of the unit cell is simply a³. Substituting the value of 'a' from step 2, we get the unit cell volume as (4r/√3)³.
-
Calculating APF: Finally, the APF is calculated by dividing the total volume of atoms by the volume of the unit cell:
APF = [2 * (4/3)πr³] / [(4r/√3)³] = (√3π)/8 ≈ 0.68
This means that in a BCC unit cell, approximately 68% of the volume is occupied by atoms. The remaining 32% is empty space.
Coordination Number and Nearest Neighbors in BCC
The coordination number in a crystal structure refers to the number of nearest neighbors surrounding a given atom. In a BCC structure, each atom is surrounded by eight nearest neighbors. These eight neighbors are located at the corners of the cube surrounding the central atom, or at the corners of the cube surrounding a corner atom. This high coordination number contributes to the strength and stability of materials with BCC structures.
Common Metals with BCC Structure
Many metals exhibit a BCC structure at certain temperatures. Some notable examples include:
- Iron (α-iron): Iron adopts a BCC structure at room temperature and up to 912°C.
- Chromium: Chromium is a BCC metal with significant industrial applications.
- Tungsten: Known for its high melting point, tungsten also possesses a BCC structure.
- Molybdenum: Another refractory metal with a BCC structure, molybdenum is used in high-temperature applications.
- Vanadium: Vanadium is a BCC metal used in various alloys due to its strength and toughness.
These metals' properties – including strength, ductility, and electrical conductivity – are strongly influenced by their BCC structure. The arrangement of atoms and the resulting APF directly impact these material characteristics.
Comparing BCC to Other Crystal Structures: FCC and SC
It's helpful to compare the BCC structure with other common crystal structures like Face-Centered Cubic (FCC) and Simple Cubic (SC) to highlight the differences in atom arrangement and packing efficiency.
-
Simple Cubic (SC): The SC structure has only one atom per unit cell, located at each corner. Its APF is significantly lower at approximately 52%. It is less common in metals due to its low packing efficiency.
-
Face-Centered Cubic (FCC): The FCC structure has atoms located at each corner and at the center of each face of the cube. It contains 4 atoms per unit cell and boasts a higher APF of approximately 74%. Many metals like aluminum, copper, and nickel exhibit FCC structures.
This comparison underscores the fact that the BCC structure, with its two atoms per unit cell and 68% APF, represents a compromise between the tightly packed FCC structure and the loosely packed SC structure.
Applications of Materials with BCC Structures
Materials exhibiting a BCC crystal structure find widespread applications across various industries due to their unique properties. These applications often leverage the strength, ductility, and other characteristics that are directly related to the BCC atomic arrangement.
-
Steel: The BCC structure of iron (α-iron) is a crucial factor determining the properties of steel. Alloying elements modify the BCC structure and influence the strength, hardness, and other mechanical properties of steel.
-
High-Temperature Alloys: Metals like molybdenum and tungsten, which are BCC at typical operating temperatures, are essential components of high-temperature alloys utilized in aerospace, power generation, and other demanding applications.
-
Nuclear Reactors: Certain BCC metals possess properties that make them suitable for use in nuclear reactors, where resistance to radiation damage and high temperatures are critical.
Beyond the Basics: Advanced Concepts Related to BCC
The discussion above primarily focuses on the ideal BCC structure. However, real-world materials can exhibit imperfections and deviations from this ideal structure. These imperfections, such as vacancies, interstitial atoms, and dislocations, influence the material's properties.
Furthermore, understanding the thermal behavior of BCC structures is critical. The temperature dependence of the BCC structure affects the material's mechanical properties and can lead to phase transformations. For instance, iron undergoes a phase transformation from BCC (α-iron) to FCC (γ-iron) at elevated temperatures.
Conclusion: The Significance of BCC Unit Cells
The number of atoms in a BCC unit cell – precisely two – is a fundamental aspect that governs the properties and applications of many crucial materials. The understanding of the BCC structure, its APF, coordination number, and its comparison with other crystal structures are essential for materials scientists, engineers, and researchers working with a broad spectrum of materials and applications. The detailed understanding of this seemingly simple aspect of material science opens doors to advancements in diverse fields.
Latest Posts
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Bcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.