How Many Asymmetric Carbons Are Present In The Compound Below
News Leon
Apr 06, 2025 · 5 min read
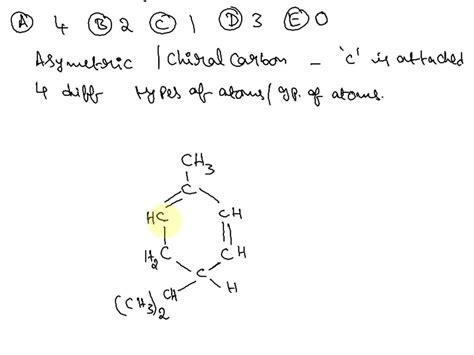
Table of Contents
How Many Asymmetric Carbons Are Present in the Compound Below? A Deep Dive into Stereochemistry
Determining the number of asymmetric carbons (also known as chiral centers) in a molecule is a fundamental concept in organic chemistry and stereochemistry. Asymmetric carbons are carbon atoms bonded to four different groups. This seemingly simple definition leads to a complex world of stereoisomers, significantly impacting a molecule's physical and biological properties. This article will delve into the process of identifying asymmetric carbons, focusing on a detailed, step-by-step approach, and offering examples to solidify understanding. We will also explore the implications of having multiple asymmetric carbons.
Understanding Asymmetric Carbons: The Foundation of Chirality
Before we embark on identifying asymmetric carbons in a specific compound, let's solidify our understanding of the fundamental principles. An asymmetric carbon, or chiral center, is a carbon atom that is bonded to four different groups. This difference can be in the form of different atoms or different arrangements of atoms. The presence of an asymmetric carbon atom is the defining characteristic of chirality, leading to the existence of enantiomers (mirror-image isomers).
Key Characteristics:
- Tetrahedral Geometry: Carbon atoms generally exhibit a tetrahedral geometry, meaning the four bonded groups are arranged in a three-dimensional tetrahedron.
- Four Different Groups: The crucial requirement for a carbon to be asymmetric is that it's bonded to four distinctly different groups.
- Non-superimposable Mirror Images: Molecules with asymmetric carbons have non-superimposable mirror images, much like your left and right hands.
Identifying Asymmetric Carbons: A Practical Approach
Let's consider a hypothetical compound to illustrate the process of identifying asymmetric carbons. Without a specific compound provided, we will use examples to clarify the method.
Example 1: 2-Bromobutane
The structural formula of 2-bromobutane is CH3CHBrCH2CH3.
-
Identify all carbon atoms: The molecule contains four carbon atoms.
-
Examine each carbon atom:
- Carbon 1 (CH3): Bonded to three hydrogens and one carbon. Not asymmetric.
- Carbon 2 (CHBr): Bonded to one methyl group (CH3), one ethyl group (CH2CH3), one hydrogen, and one bromine atom. Asymmetric.
- Carbon 3 (CH2): Bonded to two hydrogens, one carbon, and one carbon. Not asymmetric.
- Carbon 4 (CH3): Bonded to three hydrogens and one carbon. Not asymmetric.
-
Conclusion: 2-bromobutane has one asymmetric carbon (carbon 2).
Example 2: 2,3-Dibromobutane
The structural formula of 2,3-dibromobutane is CH3CHBrCHBrCH3.
-
Identify all carbon atoms: The molecule contains four carbon atoms.
-
Examine each carbon atom:
- Carbon 1 (CH3): Bonded to three hydrogens and one carbon. Not asymmetric.
- Carbon 2 (CHBr): Bonded to one methyl group (CH3), one bromomethyl group (CHBrCH3), one hydrogen, and one bromine atom. Asymmetric.
- Carbon 3 (CHBr): Bonded to one methyl group (CH3), one bromomethyl group (CHBrCH3), one hydrogen, and one bromine atom. Asymmetric.
- Carbon 4 (CH3): Bonded to three hydrogens and one carbon. Not asymmetric.
-
Conclusion: 2,3-dibromobutane has two asymmetric carbons (carbons 2 and 3).
Example 3: A More Complex Molecule (Illustrative)
Let's consider a more complex molecule, a hypothetical carbohydrate-like structure. Imagine a molecule with a six-carbon chain where carbons 2, 3, and 4 each have a hydroxyl group (-OH) and a hydrogen attached, along with other carbon attachments. A detailed analysis of each carbon would determine whether it meets the criterion of having four different groups. In a molecule of this complexity, it's advisable to draw the structure and methodically analyze each carbon. It’s possible that all three, or perhaps only one or two of carbons 2, 3 and 4, might be asymmetric. This underscores the importance of careful examination.
Implications of Multiple Asymmetric Carbons: Stereoisomers
The presence of multiple asymmetric carbons dramatically increases the number of possible stereoisomers. For a molecule with 'n' asymmetric carbons, the maximum number of stereoisomers is 2<sup>n</sup>. These stereoisomers are not necessarily all unique; some might be diastereomers (stereoisomers that are not mirror images) or enantiomers (mirror-image isomers).
Diastereomers: These are stereoisomers that differ in the configuration at one or more, but not all, chiral centers. They have different physical and chemical properties.
Enantiomers: These are stereoisomers that are non-superimposable mirror images of each other. They have identical physical properties except for their interaction with plane-polarized light and their reactions with other chiral molecules.
Further Considerations: Meso Compounds
A notable exception to the 2<sup>n</sup> rule are meso compounds. Meso compounds are molecules with multiple chiral centers but possess an internal plane of symmetry, making them achiral (not chiral). This internal symmetry cancels out the chiral effects of the individual asymmetric carbons. Identifying meso compounds requires careful analysis of molecular symmetry.
Applications and Significance
The presence and configuration of asymmetric carbons have profound implications in various fields, including:
- Pharmacology: Many drugs contain chiral centers, and the different enantiomers can exhibit significantly different pharmacological activities. One enantiomer may be therapeutically active, while the other may be inactive or even toxic.
- Biochemistry: Chiral molecules are crucial in biological systems. Enzymes, for example, often exhibit high stereoselectivity, meaning they preferentially interact with one enantiomer over the other.
- Materials Science: Chirality plays a role in the properties of materials, including their optical activity and self-assembly behavior.
Conclusion: A Critical Skill in Organic Chemistry
Determining the number of asymmetric carbons in a molecule is a fundamental skill in organic chemistry and stereochemistry. This detailed process allows for a precise understanding of a molecule's three-dimensional structure and its potential stereoisomers. The implications of this knowledge extend far beyond the classroom, impacting diverse fields such as pharmacology, biochemistry, and materials science. By mastering the identification of asymmetric carbons, we gain a deeper appreciation of the intricate world of molecular structures and their profound influence on properties and function. Remember always to carefully examine each carbon atom in a molecule, ensuring to identify all four substituents before making a conclusion. The consistent application of this method will lead to accurate determination of the number of asymmetric carbons present.
Latest Posts
Latest Posts
-
What Is Between 1 4 And 1 8
Apr 07, 2025
-
What Happens To A Plant Cell In A Hypertonic Solution
Apr 07, 2025
-
Why Are Sound Waves Called Mechanical Waves
Apr 07, 2025
-
78 Rounded To The Nearest Ten
Apr 07, 2025
-
Is Melting Wax A Chemical Or Physical Change
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Asymmetric Carbons Are Present In The Compound Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.