How Does Ka Relate To Acid Strength
News Leon
Apr 06, 2025 · 7 min read
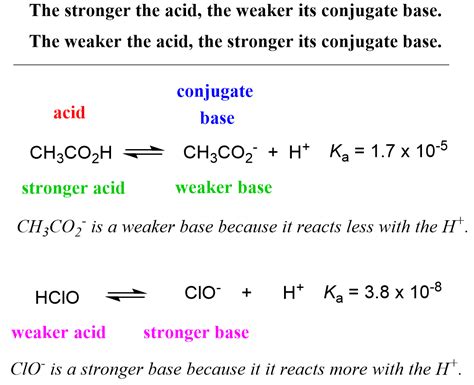
Table of Contents
How Does Ka Relate to Acid Strength? A Comprehensive Guide
Understanding the relationship between Ka and acid strength is fundamental to mastering acid-base chemistry. This comprehensive guide will delve into the intricacies of the acid dissociation constant (Ka), explaining how it quantitatively defines acid strength and how it relates to other crucial concepts like pKa and pH. We'll explore practical applications and offer examples to solidify your understanding.
What is Ka (Acid Dissociation Constant)?
The acid dissociation constant, Ka, is an equilibrium constant that quantifies the strength of an acid in solution. It represents the extent to which an acid dissociates (breaks apart) into its conjugate base and a proton (H⁺) in water. A higher Ka value indicates a stronger acid, meaning it dissociates more readily.
The general reaction for a weak acid, HA, dissociating in water is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The equilibrium expression for this reaction defines Ka:
Ka = [H⁺][A⁻] / [HA]
where:
- [H⁺] represents the concentration of hydrogen ions (protons) at equilibrium.
- [A⁻] represents the concentration of the conjugate base at equilibrium.
- [HA] represents the concentration of the undissociated acid at equilibrium.
Crucially: The concentrations are those at equilibrium, reflecting the dynamic balance between the forward (dissociation) and reverse (association) reactions.
Strong Acids vs. Weak Acids: A Ka Perspective
The magnitude of Ka dramatically differentiates strong acids from weak acids.
Strong Acids: Strong acids, like HCl (hydrochloric acid), HNO₃ (nitric acid), and H₂SO₄ (sulfuric acid), virtually completely dissociate in water. Their Ka values are very large (often > 1), indicating a near-total conversion of the acid into its conjugate base and H⁺ ions. In essence, the equilibrium lies far to the right.
Weak Acids: Weak acids, on the other hand, only partially dissociate in water. Their Ka values are significantly smaller than 1, signifying that only a small fraction of the acid molecules dissociate into ions. The equilibrium lies predominantly to the left, favoring the undissociated acid.
Examples of weak acids include acetic acid (CH₃COOH), formic acid (HCOOH), and benzoic acid (C₆H₅COOH).
pKa: A More Convenient Scale
While Ka is a valuable measure of acid strength, its numerical values can span a vast range, from very large numbers to very small ones. To simplify the comparison of acid strengths, the pKa scale is used. pKa is defined as the negative logarithm (base 10) of Ka:
pKa = -log₁₀(Ka)
This logarithmic transformation converts the wide range of Ka values into a more manageable scale. A lower pKa value indicates a stronger acid. For example, an acid with a pKa of 2 is stronger than an acid with a pKa of 5.
The relationship between Ka and pKa is inverse:
- Higher Ka = Lower pKa = Stronger Acid
- Lower Ka = Higher pKa = Weaker Acid
Factors Affecting Ka and Acid Strength
Several factors influence the Ka value and therefore the acid strength:
1. Bond Strength:
The strength of the bond between the acidic hydrogen (H⁺) and the rest of the molecule plays a significant role. Weaker bonds lead to easier dissociation and thus a higher Ka. For example, the weaker O-H bond in carboxylic acids compared to the stronger O-H bond in alcohols explains the higher acidity of carboxylic acids.
2. Electronegativity:
The electronegativity of the atom bonded to the acidic hydrogen influences the polarity of the bond. A more electronegative atom pulls electron density away from the hydrogen, weakening the bond and making it easier to donate a proton. This results in a higher Ka. The presence of electronegative atoms like oxygen, chlorine, or fluorine near the acidic hydrogen significantly increases acid strength.
3. Resonance:
Resonance stabilization of the conjugate base significantly impacts acid strength. If the conjugate base can be stabilized through resonance, the overall stability of the products of dissociation increases, pushing the equilibrium towards dissociation (higher Ka). Carboxylic acids exhibit this resonance stabilization, explaining their comparatively higher acidity than alcohols.
4. Inductive Effects:
Electron-withdrawing groups (-I effect) increase the acidity by pulling electron density away from the acidic hydrogen, thereby weakening the bond. Conversely, electron-donating groups (+I effect) decrease acidity. The presence of electron-withdrawing groups near the acidic hydrogen increases the Ka.
5. Hybridization:
The hybridization of the atom bonded to the acidic hydrogen also plays a role. More s-character in the hybrid orbital leads to a stronger bond and lower Ka. For instance, sp-hybridized carbon in terminal alkynes results in a weaker acid compared to sp²-hybridized carbon in alkenes.
Ka and pH: The Interplay
Ka is directly related to the pH of a solution of a weak acid. The pH, a measure of hydrogen ion concentration, can be calculated using the Ka value if the initial concentration of the acid is known. This is typically done using an ICE (Initial, Change, Equilibrium) table for weak acid dissociation.
For a weak acid, HA, with initial concentration [HA]₀, we can approximate the pH using the following equation (assuming the x is small approximation):
[H⁺] ≈ √(Ka[HA]₀)
Then, the pH can be calculated as:
pH = -log₁₀[H⁺]
This calculation is simplified by the assumption that the concentration of H⁺ is significantly smaller than the initial concentration of the acid. This assumption is valid when Ka is small and the initial concentration of the acid is relatively high. However, for more precise calculations, the quadratic formula must be used to solve the equilibrium expression.
Practical Applications of Ka
The understanding of Ka and its relationship to acid strength has wide-ranging applications in various fields:
- Chemistry: Predicting reaction outcomes in acid-base reactions, designing buffer solutions, titrations, and understanding equilibrium processes.
- Biology: Determining the pH of biological systems, understanding enzyme activity (which is often pH-dependent), and studying the role of acids and bases in metabolic processes.
- Medicine: Formulating drugs, controlling pH in pharmaceutical preparations, and understanding the action of acid-base regulators in the body.
- Environmental Science: Monitoring water quality, assessing soil acidity, and understanding acid rain's effects on ecosystems.
- Industrial Chemistry: Controlling reaction conditions, designing catalysts, and selecting appropriate solvents in various industrial processes.
Solving Problems Involving Ka
Let's illustrate the practical use of Ka with a few examples:
Example 1: Calculating the pH of a weak acid solution.
Calculate the pH of a 0.1 M solution of acetic acid (CH₃COOH) with Ka = 1.8 x 10⁻⁵.
Using the simplified equation (assuming the x is small approximation):
[H⁺] ≈ √(1.8 x 10⁻⁵ x 0.1) ≈ 1.34 x 10⁻³ M
pH = -log₁₀(1.34 x 10⁻³) ≈ 2.87
Example 2: Comparing the relative strengths of two acids.
Acid A has a Ka of 10⁻⁴, and Acid B has a Ka of 10⁻⁶. Which acid is stronger?
Since Acid A has a higher Ka value, it is the stronger acid. Alternatively, Acid A has a lower pKa (pKa = 4) than Acid B (pKa = 6).
Example 3: Determining the Ka from pH and concentration.
A 0.05 M solution of a weak acid has a pH of 3.5. What is the Ka of this acid?
First, calculate the [H⁺]:
[H⁺] = 10⁻³·⁵ ≈ 3.16 x 10⁻⁴ M
Then, use the equilibrium expression for Ka:
Ka = [H⁺][A⁻]/[HA] (Assuming [H⁺] ≈ [A⁻])
Ka ≈ (3.16 x 10⁻⁴)² / (0.05 - 3.16 x 10⁻⁴) ≈ 2.0 x 10⁻⁶
These examples demonstrate how Ka is used to quantitatively determine and compare acid strengths and calculate solution pH. Mastering these concepts is crucial for a deep understanding of acid-base chemistry.
Conclusion: Ka – The Key to Understanding Acid Strength
The acid dissociation constant, Ka, and its logarithmic counterpart, pKa, are indispensable tools for understanding and quantifying acid strength. The higher the Ka (or lower the pKa), the stronger the acid. Numerous factors influence Ka, including bond strength, electronegativity, resonance, inductive effects, and hybridization. The interplay between Ka and pH allows for calculations of hydrogen ion concentrations in solutions, and this knowledge is crucial across various scientific disciplines and practical applications. Through a combination of theoretical understanding and problem-solving practice, one can master the crucial relationship between Ka and acid strength.
Latest Posts
Latest Posts
-
The Resources A Business Owns Are Called
Apr 08, 2025
-
What Element Has 4 Neutrons And 3 Protons
Apr 08, 2025
-
What Is The Phylum Of A Crab
Apr 08, 2025
-
A Negative Income Elasticity Of Demand Indicates That The Product
Apr 08, 2025
-
Which Of The Following Pairs Is Correct
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How Does Ka Relate To Acid Strength . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.