H3po4 Mg Oh 2 Mg3 Po4 2 H2o
News Leon
Apr 07, 2025 · 5 min read
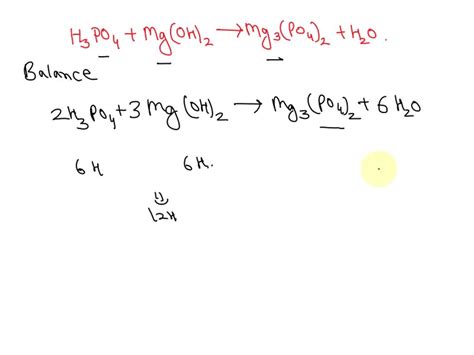
Table of Contents
Delving Deep into the Reaction Between H3PO4, Mg(OH)2, and the Formation of Mg3(PO4)2·2H2O
This article explores the chemical reaction between phosphoric acid (H3PO4) and magnesium hydroxide [Mg(OH)2], focusing on the formation of magnesium phosphate dihydrate [Mg3(PO4)2·2H2O]. We'll delve into the stoichiometry, reaction mechanism, applications, and industrial significance of this important chemical process.
Understanding the Reactants
Before diving into the reaction itself, let's briefly examine the properties of the reactants: phosphoric acid and magnesium hydroxide.
Phosphoric Acid (H3PO4)
Phosphoric acid, also known as orthophosphoric acid, is a weak, non-toxic, and odorless mineral acid. Its chemical formula is H3PO4. It's a triprotic acid, meaning it can donate three protons (H+) in aqueous solutions. The successive dissociation constants (Ka) indicate its relatively weak acidity compared to strong mineral acids like sulfuric or hydrochloric acid.
Key Properties of H3PO4:
- Molecular Weight: Approximately 97.99 g/mol
- Appearance: Colorless, syrupy liquid
- Solubility: Highly soluble in water
- Applications: Widely used in fertilizers, food additives (as an acidity regulator), detergents, and various industrial processes.
Magnesium Hydroxide [Mg(OH)2]
Magnesium hydroxide is a white, crystalline solid with low solubility in water. It's a mild alkali and a common component of antacids and laxatives due to its ability to neutralize stomach acid.
Key Properties of Mg(OH)2:
- Molecular Weight: Approximately 58.32 g/mol
- Appearance: White crystalline powder
- Solubility: Slightly soluble in water
- Applications: Antacids, laxatives, fire retardants, and in the production of magnesium compounds.
The Reaction: H3PO4 + Mg(OH)2 → Mg3(PO4)2·2H2O
The reaction between phosphoric acid and magnesium hydroxide is a classic acid-base neutralization reaction resulting in the formation of magnesium phosphate dihydrate (Mg3(PO4)2·2H2O) and water. The balanced chemical equation is:
2H3PO4 + 3Mg(OH)2 → Mg3(PO4)2·2H2O + 4H2O
This equation shows that two moles of phosphoric acid react with three moles of magnesium hydroxide to produce one mole of magnesium phosphate dihydrate and four moles of water. The reaction is exothermic, meaning it releases heat.
Reaction Mechanism
The reaction proceeds in stages. First, the hydroxide ions (OH-) from magnesium hydroxide react with the protons (H+) from phosphoric acid. This neutralization step forms water and magnesium phosphate.
Stage 1: Neutralization
H3PO4 + OH- → H2PO4- + H2O H2PO4- + OH- → HPO42- + H2O HPO42- + OH- → PO43- + H2O
Stage 2: Precipitation
Three magnesium cations (Mg2+) combine with two phosphate anions (PO43-) to form insoluble magnesium phosphate. The low solubility of magnesium phosphate leads to its precipitation out of solution.
3Mg2+ + 2PO43- → Mg3(PO4)2
Stage 3: Hydration
The precipitated magnesium phosphate incorporates two molecules of water into its crystal structure, forming the dihydrate Mg3(PO4)2·2H2O. This hydration process is often dependent on the reaction conditions, such as temperature and concentration.
Factors Affecting the Reaction
Several factors influence the reaction rate and yield of Mg3(PO4)2·2H2O:
- Concentration of Reactants: Higher concentrations of both H3PO4 and Mg(OH)2 generally lead to a faster reaction rate.
- Temperature: Increasing the temperature usually accelerates the reaction, but excessively high temperatures might lead to undesirable side reactions.
- pH: Controlling the pH of the reaction mixture is crucial to ensure the complete precipitation of Mg3(PO4)2.
- Mixing: Proper mixing of the reactants ensures that the reactants are in constant contact, promoting a faster reaction rate.
- Presence of Impurities: The presence of certain impurities in the reactants or the reaction medium can affect the reaction rate and the purity of the final product.
Applications of Mg3(PO4)2·2H2O
Magnesium phosphate dihydrate finds various applications across different industries:
- Fertilizers: It's a valuable source of phosphorus and magnesium, essential nutrients for plant growth. Its slow-release nature makes it an attractive choice for sustainable agriculture.
- Flame Retardants: Mg3(PO4)2·2H2O exhibits excellent flame-retardant properties and is incorporated into various materials to improve their fire safety.
- Dental Cements: Its biocompatibility and setting properties make it suitable for use in dental cements and restorative materials.
- Water Treatment: It can be used in water treatment processes to remove heavy metals and other impurities.
- Food Additives: While less common than other phosphate salts, it can find limited applications in food processing as a stabilizer or additive.
Industrial Production of Mg3(PO4)2·2H2O
The industrial production of Mg3(PO4)2·2H2O typically involves reacting phosphoric acid with a magnesium-containing compound, such as magnesium oxide (MgO) or magnesium hydroxide [Mg(OH)2]. The process often involves careful control of reaction parameters like temperature, pH, and concentration to achieve high purity and yield. The resulting precipitate is then filtered, washed, and dried to obtain the final product. Various techniques, including crystallization and precipitation from controlled solutions, are used to optimize the process and improve the quality of the magnesium phosphate dihydrate.
Safety Precautions
When handling phosphoric acid and magnesium hydroxide, appropriate safety measures are essential. Phosphoric acid can cause skin and eye irritation, while magnesium hydroxide, while less hazardous, can still cause mild skin and eye irritation. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and lab coats when handling these chemicals. Proper ventilation is also necessary to minimize exposure to any potential fumes or dust.
Conclusion
The reaction between H3PO4 and Mg(OH)2 to produce Mg3(PO4)2·2H2O is a significant chemical process with broad applications across various industries. Understanding the reaction mechanism, influencing factors, and industrial production techniques is crucial for optimizing its use and maximizing its benefits. Further research continues to explore the potential of magnesium phosphate dihydrate in emerging fields such as biomaterials and advanced materials science. The versatility and important properties of this compound make it a valuable material with continued relevance for technological advancements and industrial applications. This detailed exploration highlights the importance of this seemingly simple chemical reaction and its remarkable impact on various aspects of modern life.
Latest Posts
Latest Posts
-
How To Write A Conjecture In Math
Apr 08, 2025
-
Which Of The Following Is A Good Conductor Of Heat
Apr 08, 2025
-
Is Gas Evaporating A Chemical Change
Apr 08, 2025
-
Graph Of Kinetic Energy Vs Time
Apr 08, 2025
-
Which Of The Following Is Correctly Paired
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about H3po4 Mg Oh 2 Mg3 Po4 2 H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.