Give The Complete Electron Configuration For Mn
News Leon
Apr 06, 2025 · 6 min read
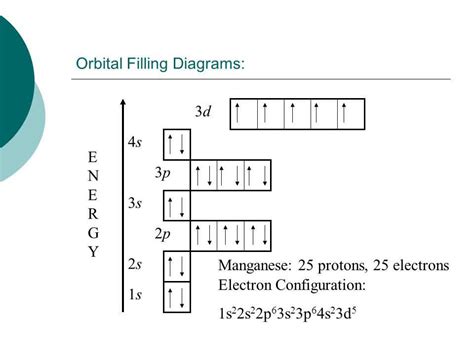
Table of Contents
The Complete Electron Configuration for Manganese (Mn) and its Implications
Manganese (Mn), a transition metal with atomic number 25, boasts a fascinating electron configuration that significantly influences its chemical and physical properties. Understanding this configuration is crucial for comprehending its role in various biological processes, industrial applications, and its unique behavior in different chemical environments. This article delves deep into the electron configuration of manganese, explaining its derivation, implications, and the subtle nuances that shape its reactivity and characteristics.
Understanding Electron Configuration
Before we delve into the specifics of manganese's electron configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement follows specific rules, primarily governed by the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
- Aufbau principle: Electrons fill the lowest energy levels first. Think of it like filling a building from the ground floor up.
- Hund's rule: Within a sublevel (like a p or d orbital), electrons will occupy each orbital singly before pairing up. This minimizes electron-electron repulsion. Imagine each seat in a row being filled before doubling up.
- Pauli exclusion principle: No two electrons within an atom can have the same set of four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons with opposite spins.
These principles dictate the order in which electrons occupy the various orbitals, resulting in a unique electron configuration for each element.
Deriving the Electron Configuration of Manganese (Mn)
Manganese, with an atomic number of 25, has 25 electrons. Following the Aufbau principle, we systematically fill the energy levels and sublevels:
- 1s²: The first energy level (n=1) contains only the s sublevel, which can hold a maximum of two electrons.
- 2s² 2p⁶: The second energy level (n=2) contains an s sublevel (holding two electrons) and a p sublevel (holding six electrons).
- 3s² 3p⁶: The third energy level (n=3) similarly contains an s sublevel (two electrons) and a p sublevel (six electrons).
- 4s² 3d⁵: This is where things get interesting. The fourth energy level starts filling with the 4s sublevel (two electrons). Then, the 3d sublevel begins to fill. Note: While the 4s sublevel fills before the 3d, the 3d sublevel is actually at a slightly higher energy level than the 4s. This is a slight exception to a simple Aufbau order, caused by complex electron-electron interactions. The 3d sublevel can hold up to ten electrons, and in manganese, it has five electrons. Importantly, Hund's rule dictates that these five electrons occupy five separate 3d orbitals singly, before pairing up. This gives manganese a half-filled 3d subshell, which is a particularly stable electron configuration.
Therefore, the complete electron configuration for manganese is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵.
The Significance of the Half-Filled 3d Subshell
The half-filled 3d subshell in manganese is a key factor influencing its properties. A half-filled or fully-filled subshell represents a state of enhanced stability due to exchange energy. Electrons with parallel spins in separate orbitals experience a lower repulsive energy and are more stable than electrons paired in the same orbital. This extra stability contributes to manganese's relatively high ionization energies and its tendency to exhibit multiple oxidation states.
Manganese's Variable Oxidation States
The relatively easy removal or sharing of electrons from the 4s and 3d subshells is a direct consequence of its electron configuration. Manganese exhibits numerous oxidation states, ranging from +2 to +7. This versatility makes it an important element in many chemical reactions and catalytic processes. Some examples of manganese's different oxidation states and their corresponding compounds include:
- Mn²⁺ (Manganese(II)): Found in compounds like manganese(II) sulfate (MnSO₄). Relatively stable and common.
- Mn³⁺ (Manganese(III)): Found in compounds like manganese(III) oxide (Mn₂O₃). Less stable than Mn²⁺.
- Mn⁴⁺ (Manganese(IV)): Found in manganese dioxide (MnO₂), a crucial component in batteries and as a catalyst.
- Mn⁷⁺ (Manganese(VII)): Found in potassium permanganate (KMnO₄), a strong oxidizing agent used in various applications, including titrations and disinfectants.
Each oxidation state possesses unique chemical reactivity, contributing to the rich chemistry of manganese.
Applications of Manganese and its Electron Configuration
The unique electron configuration and subsequent properties of manganese make it indispensable in various fields:
- Steel Production: Manganese is a crucial alloying element in steel production. It improves the steel's strength, hardness, and toughness, making it ideal for various structural applications. Its ability to readily form carbides also influences the steel's microstructure.
- Batteries: Manganese dioxide (MnO₂) is a key component in many battery systems, particularly alkaline batteries and some rechargeable batteries. Its ability to readily accept and release electrons contributes to the battery's performance.
- Pigments: Manganese compounds are utilized as pigments in paints, ceramics, and other materials. Different manganese oxidation states produce distinct colors, providing a wide range of options for color customization.
- Catalysis: Manganese compounds act as catalysts in various industrial processes, such as the production of chemicals and the oxidation of organic compounds. Their variable oxidation states and ability to participate in redox reactions are key to their catalytic capabilities.
- Biological Roles: Manganese plays vital roles in several biological processes. It is a cofactor in various enzymes involved in photosynthesis, oxygen metabolism, and bone formation. The specific oxidation state and coordination environment of manganese in these enzymes influence their activity.
Beyond the Basic Configuration: Orbital Diagrams and Quantum Numbers
While the electron configuration provides a general overview, a more detailed picture emerges when we consider orbital diagrams and quantum numbers.
An orbital diagram uses boxes to represent orbitals and arrows to represent electrons, illustrating how electrons fill orbitals within a subshell. For manganese's 3d subshell, an orbital diagram visually demonstrates the five unpaired electrons occupying individual orbitals, consistent with Hund's rule.
Each electron is characterized by four quantum numbers:
- Principal quantum number (n): Indicates the energy level (1, 2, 3, etc.).
- Azimuthal quantum number (l): Indicates the sublevel (0 for s, 1 for p, 2 for d, 3 for f).
- Magnetic quantum number (ml): Indicates the specific orbital within a sublevel (-l to +l).
- Spin quantum number (ms): Indicates the electron's spin (+1/2 or -1/2).
These quantum numbers provide a precise description of each electron's state within the manganese atom.
Conclusion: The Impact of Electron Configuration
The complete electron configuration of manganese (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵) is not just a list of numbers; it is a fundamental blueprint that dictates its chemical and physical behavior. The half-filled 3d subshell, the variable oxidation states, and the resulting diverse applications showcase the significance of understanding electron configurations in the context of atomic structure and reactivity. This deep dive into manganese’s electronic structure highlights the power of quantum mechanics in predicting and explaining the macroscopic properties of matter. Further exploration into the intricacies of manganese's electronic structure through advanced spectroscopic techniques continually refines our understanding of this crucial element.
Latest Posts
Latest Posts
-
Find The Area Of The Number 7
Apr 09, 2025
-
An Ion That Has A Negative Charge
Apr 09, 2025
-
The Act Of White Blood Cell Engulfing A Bacterium Is
Apr 09, 2025
-
What Do Transverse And Longitudinal Waves Have In Common
Apr 09, 2025
-
What Are The Units Of Potential Energy
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Give The Complete Electron Configuration For Mn . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.