Freezing Point For Water In Kelvin
News Leon
Mar 18, 2025 · 6 min read
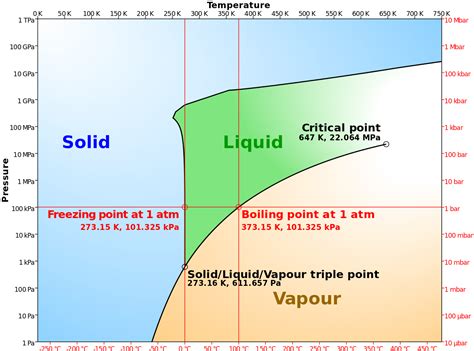
Table of Contents
Freezing Point of Water in Kelvin: A Deep Dive into Thermodynamics and its Applications
The freezing point of water, a seemingly simple concept, holds significant importance across numerous scientific disciplines and everyday life. Understanding this fundamental property, especially when expressed in Kelvin, unlocks a deeper understanding of thermodynamics, phase transitions, and their practical implications. This article will delve into the intricacies of water's freezing point in Kelvin, exploring its definition, the science behind it, and its relevance in various fields.
Defining the Freezing Point
The freezing point of a substance is the temperature at which it transitions from a liquid state to a solid state. For pure water under standard atmospheric pressure (1 atmosphere or 101.325 kPa), this transition occurs at 0 degrees Celsius (°C). However, expressing this temperature in Kelvin (K) provides a more fundamental and scientifically relevant representation.
The Kelvin scale, an absolute temperature scale, is defined by assigning 0 K to absolute zero—the theoretical temperature at which all molecular motion ceases. The relationship between Celsius and Kelvin is straightforward:
K = °C + 273.15
Therefore, the freezing point of water in Kelvin is:
0 °C + 273.15 = 273.15 K
The Science Behind Water's Freezing Point
Water's freezing point is not merely a number; it's a consequence of the intricate interplay of intermolecular forces and molecular structure. Water molecules (H₂O) are polar, meaning they possess a slightly positive end (hydrogen atoms) and a slightly negative end (oxygen atom). This polarity leads to strong hydrogen bonds between water molecules.
Hydrogen Bonding and Crystallization
As water cools, its kinetic energy decreases. The molecules move more slowly, allowing the hydrogen bonds to become more prominent. Below 0°C (273.15 K), these hydrogen bonds overcome the kinetic energy of the molecules, forcing them into a highly ordered, crystalline structure—ice. This crystallization process releases latent heat, which is why the temperature remains constant during the phase transition.
The unique hexagonal structure of ice is crucial to its properties. This structure is less dense than liquid water, which is why ice floats on water. This seemingly minor detail has profound implications for aquatic life and the Earth's climate.
Factors Affecting the Freezing Point
While 273.15 K is the freezing point of pure water under standard conditions, several factors can influence this temperature:
-
Pressure: Increasing pressure slightly lowers the freezing point of water. This is an unusual property, stemming from the lower density of ice compared to liquid water. At very high pressures, different ice polymorphs (different crystal structures) can form, each with its own freezing point.
-
Impurities: Dissolving solutes (like salt) in water lowers its freezing point. This phenomenon, known as freezing point depression, is a colligative property, meaning it depends on the concentration of solute particles, not their identity. This principle is exploited in various applications, such as de-icing roads and preserving food.
-
Isotopes: The isotopic composition of water can slightly affect its freezing point. Water enriched in heavier isotopes (like deuterium) has a slightly higher freezing point than water composed primarily of lighter isotopes.
-
Supercooling: Under certain conditions, water can be cooled below its freezing point without solidifying. This phenomenon, known as supercooling, requires a very clean environment and the absence of nucleation sites (surfaces or impurities that facilitate crystallization). Supercooled water is metastable, meaning it's in a temporarily stable state and will quickly freeze upon disturbance.
Applications and Relevance of Water's Freezing Point
The freezing point of water, particularly its precise value in Kelvin, is crucial across various scientific and practical fields:
1. Calorimetry and Thermodynamics:
Precise measurements of the freezing point of water are essential for calibrating thermometers and other instruments used in calorimetry (the study of heat transfer). The enthalpy of fusion (heat required to melt ice) is also determined relative to the freezing point, providing a fundamental thermodynamic property of water.
2. Cryobiology:
Cryobiology, the study of the effects of low temperatures on living organisms, relies heavily on understanding the freezing point of water. Freezing can damage cells and tissues due to ice crystal formation. Cryobiologists utilize various techniques to mitigate this damage, including cryoprotective agents that lower the freezing point and reduce ice crystal formation.
3. Meteorology and Climatology:
The freezing point of water is a critical parameter in meteorology and climatology. Understanding how temperature changes influence the formation of ice, snow, and other forms of precipitation is essential for weather forecasting and climate modeling. The phase transitions of water play a central role in the Earth's climate system.
4. Food Science and Preservation:
Freezing is a widely used food preservation method. Controlling the freezing rate and temperature is crucial to maintain food quality. The freezing point of water, especially considering the effects of solutes, is a key factor in determining the effectiveness of freezing as a preservation technique.
5. Material Science and Engineering:
The freezing point of water impacts numerous materials and engineering processes. For example, the formation of ice can cause damage to infrastructure during freezing winters. Understanding the freezing point and its relation to pressure is important in designing structures resistant to freezing damage.
6. Environmental Science and Hydrology:
The freezing and thawing of water significantly impact ecosystems and hydrological cycles. The freezing of water bodies can affect aquatic life, alter water flow patterns, and contribute to soil erosion. Understanding the processes involved requires accurate knowledge of water's freezing point.
7. Chemistry and Biochemistry:
Water's freezing point is fundamental to many chemical and biochemical processes. Many reactions are affected by temperature, and the freezing point provides a key reference point. In biochemistry, studying protein folding and other biological processes often requires precise temperature control around the freezing point of water.
Beyond the Basics: Exploring Anomalies and Further Research
While 273.15 K represents the standard freezing point of water, the reality is far richer and more complex. Ongoing research continues to refine our understanding of water's properties at and near its freezing point:
-
Investigations into supercooled water: Understanding the behavior of supercooled water and the mechanisms that initiate freezing are areas of ongoing research. This has implications for atmospheric science, as supercooled water droplets are crucial in cloud formation.
-
Studies of different ice polymorphs: Beyond the common hexagonal ice (Ice Ih), several other ice polymorphs exist, each with unique properties and freezing points at different pressures. Research continues to elucidate the behaviour of these various ice phases and their potential applications.
-
Advanced techniques for measuring freezing point: Researchers are constantly developing more precise methods for measuring the freezing point of water, allowing for more accurate measurements and deeper insight into the underlying physics.
Conclusion
The freezing point of water in Kelvin, 273.15 K, is far more than a simple numerical value. It is a cornerstone of thermodynamics, a key parameter in numerous scientific disciplines, and a crucial factor in various everyday processes. Understanding this fundamental property, along with the factors that can influence it, is vital for advances in science, technology, and our understanding of the world around us. As research continues, we can expect even greater insights into the intricate behavior of water at its freezing point and its significance in diverse fields. The seemingly simple freezing of water reveals a complex interplay of forces, a testament to the rich and ever-evolving nature of scientific inquiry.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point For Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
