Example Of Expanded Octet Molecule Is
News Leon
Mar 26, 2025 · 5 min read
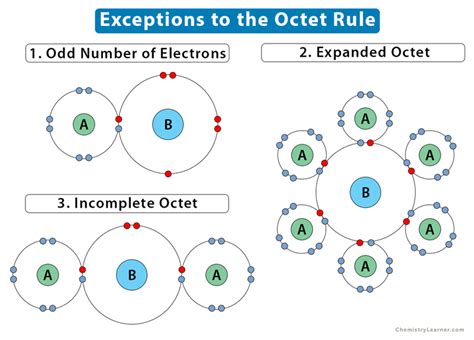
Table of Contents
Examples of Expanded Octet Molecules: Beyond the Octet Rule
The octet rule, a cornerstone of introductory chemistry, dictates that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons. However, this rule isn't absolute. Many molecules, particularly those involving elements from the third period and beyond, readily violate the octet rule, exhibiting what's known as an expanded octet. This article delves into the fascinating world of expanded octet molecules, exploring their structures, bonding characteristics, and the reasons behind their ability to accommodate more than eight valence electrons.
Understanding the Limitations of the Octet Rule
The octet rule stems from the stability associated with filled s and p orbitals, resulting in a noble gas electron configuration. Elements in the second period (Li to Ne) strictly adhere to this rule due to their limited number of valence orbitals. However, elements in the third period and beyond possess accessible d orbitals. These d orbitals can participate in bonding, allowing these atoms to accommodate more than eight electrons in their valence shell. This is the key to understanding expanded octet molecules.
Why do Expanded Octets Occur?
The expansion of the octet is primarily driven by the availability of low-energy d orbitals. These orbitals can participate in bonding, accepting additional electron pairs beyond the eight electrons that would fill the s and p orbitals. This phenomenon is particularly common in compounds involving elements such as phosphorus, sulfur, silicon, chlorine, and bromine. The energy difference between the valence shell and the d orbitals is relatively small for these elements, making the participation of d orbitals energetically favorable.
Furthermore, the electronegativity of the central atom and the surrounding ligands also plays a crucial role. Highly electronegative ligands can effectively withdraw electron density from the central atom, reducing electron-electron repulsion and making the expansion of the octet more feasible.
Key Examples of Expanded Octet Molecules
Let's explore some illustrative examples of molecules that exhibit expanded octets:
1. Phosphorus Pentachloride (PCl₅)
Phosphorus pentachloride is a classic example. Phosphorus, having five valence electrons, cannot satisfy its bonding requirements with just four bonds (as it would in PCl3 which follows octet rule), as that would leave one unbonded electron. In PCl₅, phosphorus forms five bonds with five chlorine atoms, resulting in ten valence electrons surrounding the central phosphorus atom – a clear violation of the octet rule. This expansion is achieved by utilizing its 3d orbitals. The molecule adopts a trigonal bipyramidal geometry.
2. Sulfur Hexafluoride (SF₆)
Sulfur hexafluoride is another well-known example, featuring sulfur bonded to six fluorine atoms. Sulfur, with six valence electrons, expands its octet to accommodate twelve electrons, resulting in a stable molecule. The high electronegativity of fluorine assists in this expansion by drawing electron density away from the sulfur atom, mitigating electron-electron repulsion. The molecule exhibits octahedral geometry.
3. Xenon Tetrafluoride (XeF₄)
Xenon tetrafluoride, a noble gas compound, showcases the expanded octet concept beyond typical elements. Xenon, having eight valence electrons in its ground state, surpasses the octet rule by forming four bonds with four fluorine atoms. Two lone pairs of electrons also reside on the xenon atom, leading to a total of 12 electrons. The molecule possesses a square planar geometry.
4. Phosphoric Acid (H₃PO₄)
Phosphoric acid, a crucial compound in many biological and industrial processes, features phosphorus exhibiting an expanded octet. Phosphorus forms four bonds with oxygen atoms (one P=O double bond and three P-O single bonds), and the oxygen atoms are further bonded to hydrogen atoms. Phosphorus therefore has 10 valence electrons surrounding it.
5. Silicon Tetrachloride (SiCl₄)
Silicon tetrachloride exemplifies expanded octet formation in silicon compounds. Silicon, with four valence electrons, readily forms four bonds with chlorine atoms, creating a tetrahedral structure. While this might seem to satisfy the octet rule, many chemists consider this to be a hypervalent molecule, where the presence of 3d orbitals allows for some degree of hypervalence.
6. Iodine Pentafluoride (IF₅)
Iodine pentafluoride displays an expanded octet, with iodine, despite having seven valence electrons, expanding to accommodate ten electrons. It features one lone pair and five bonds, resulting in a square pyramidal molecular geometry. The high electronegativity of fluorine contributes to this expansion.
7. Chlorine Trifluoride (ClF₃)
Chlorine trifluoride is a hypervalent molecule where the central chlorine atom bonds to three fluorine atoms, with two lone pairs on the chlorine atom. This leads to a total of ten electrons around chlorine, significantly exceeding the octet. The high electronegativity of fluorine plays a critical role here.
Hypervalency vs. Expanded Octet: A Subtle Distinction
The terms "expanded octet" and "hypervalency" are often used interchangeably, but a subtle difference exists. Hypervalency refers to any molecule where the central atom has more than eight valence electrons. This broader term encompasses molecules with expanded octets. While d orbital participation is commonly cited as the reason for expanded octets, the debate continues regarding the true extent of d orbital involvement in hypervalent bonding. Alternative bonding models, such as three-center four-electron bonds, are also proposed to explain hypervalency.
Predicting Expanded Octets
While no single rule perfectly predicts expanded octets, some guidelines prove helpful:
- Period: Elements in the third period and beyond are more likely to exhibit expanded octets.
- Electronegativity: Highly electronegative ligands promote octet expansion.
- Central atom: The central atom's ability to accommodate additional electron pairs.
Consequences of Expanded Octets
Expanded octets lead to distinct molecular geometries, influencing the properties of these compounds. For instance, the geometry of SF₆ (octahedral) drastically differs from that of similar molecules following the octet rule. The presence of expanded octets can also impact reactivity and stability.
Beyond the Examples: Applications and Significance
The understanding of expanded octet molecules is essential in various fields:
- Inorganic Chemistry: Understanding bonding in these compounds is crucial for synthesizing new materials.
- Materials Science: Many materials with unique properties, such as high thermal stability and reactivity, contain expanded octet molecules.
- Catalysis: Several catalysts involve compounds with expanded octets, influencing reaction mechanisms and selectivity.
- Environmental Chemistry: The study of expanded octet molecules aids in understanding atmospheric processes and pollution control.
Conclusion
Expanded octet molecules challenge the traditional octet rule, demonstrating the flexibility of chemical bonding. Their existence underscores the importance of considering factors beyond simple electron counting. Understanding the principles governing expanded octets is crucial for comprehending the structure, reactivity, and properties of a wide range of molecules with significant implications across scientific disciplines. Further research into the bonding mechanisms involved in hypervalent molecules continues to be an active area of study in chemistry.
Latest Posts
Latest Posts
-
Which Of The Following Bodies Has The Largest Kinetic Energy
Mar 29, 2025
-
A Cell That Contains One Set Of Chromosomes
Mar 29, 2025
-
An Electric Vehicle Starts From Rest And Accelerates
Mar 29, 2025
-
Chemical Behavior Of An Atom Is Determined By
Mar 29, 2025
-
When Elements Combine To Form Compounds
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Example Of Expanded Octet Molecule Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
