Enzymes Are Catalysts That Increase The Rate Of Reactions By
News Leon
Apr 04, 2025 · 8 min read
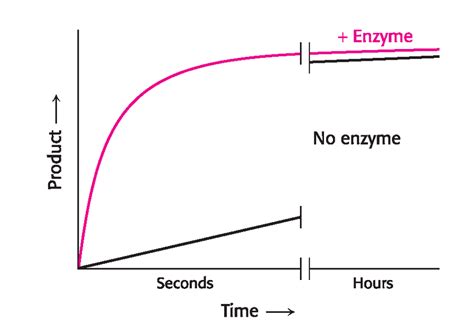
Table of Contents
Enzymes: Catalysts That Supercharge Biological Reactions
Enzymes are remarkable biological molecules that act as catalysts, dramatically increasing the rate of virtually all chemical reactions within cells. Without enzymes, life as we know it would be impossible. These biological workhorses facilitate countless processes essential for survival, from digestion and respiration to DNA replication and protein synthesis. Understanding how enzymes achieve this catalytic power is fundamental to comprehending the intricate workings of living organisms.
The Nature of Enzyme Catalysis
The fundamental principle behind enzyme catalysis lies in their ability to lower the activation energy of a reaction. Activation energy is the minimum energy required for a reaction to proceed. Think of it as the "energy hill" reactants must overcome to transform into products. Enzymes act as molecular "tunnels" through this energy hill, creating a lower-energy pathway that requires less energy input to initiate the reaction. This significant reduction in activation energy accelerates the reaction rate, often by many orders of magnitude.
How Enzymes Lower Activation Energy: A Closer Look
Several mechanisms contribute to enzymes' remarkable ability to lower activation energy:
-
Substrate Binding and Orientation: Enzymes possess a specific region called the active site, which is precisely shaped to bind to the reactants (called substrates). This binding process brings the substrates into close proximity and proper orientation, maximizing the likelihood of a successful reaction. This is akin to holding two puzzle pieces together, making it easier to connect them.
-
Strain and Distortion of Substrates: The enzyme's active site can induce strain or distortion in the substrate molecules. This destabilization of the substrate makes it easier to break existing bonds and form new ones. It’s like bending a stick until it's about to break, requiring less force to snap it.
-
Acid-Base Catalysis: Certain amino acid residues within the active site can act as acids or bases, donating or accepting protons (H⁺) to facilitate bond formation or breakage. This precise proton transfer is crucial for many enzymatic reactions. It’s analogous to using a tool to help separate components.
-
Covalent Catalysis: In some cases, the enzyme forms a temporary covalent bond with the substrate. This covalent intermediate then undergoes further reactions to generate the product, after which the enzyme reverts to its original state. Think of it as a temporary glue helping join two parts.
-
Metal Ion Catalysis: Many enzymes require metal ions (like zinc, magnesium, or iron) for their activity. These ions participate in various ways, including stabilizing reaction intermediates, facilitating redox reactions, or enhancing substrate binding. They are like the key that unlocks the potential of the enzyme.
Enzyme Specificity: A Lock and Key Mechanism
One of the defining characteristics of enzymes is their remarkable specificity. Each enzyme typically catalyzes only one specific type of reaction, or a very small number of closely related reactions. This specificity stems from the highly precise structure of the active site, which acts like a "lock" that only fits a specific "key" (the substrate). This is often referred to as the lock-and-key model, though a more accurate representation is the induced-fit model.
The Induced-Fit Model: A Dynamic Interaction
The induced-fit model recognizes that the enzyme's active site is not a rigid structure but rather a flexible one. Upon substrate binding, the enzyme undergoes a conformational change, adapting its shape to optimally interact with the substrate. This dynamic interaction enhances the catalytic efficiency by precisely aligning the reactive groups of the substrate and enzyme. It's like a glove adjusting to the shape of your hand, creating a perfect fit.
Factors Affecting Enzyme Activity
Several factors influence the rate at which enzymes catalyze reactions. Understanding these factors is crucial for optimizing enzyme activity in various applications, from industrial processes to medical treatments.
Substrate Concentration: The Saturation Point
At low substrate concentrations, the reaction rate increases proportionally to the substrate concentration. As the substrate concentration increases, the enzyme active sites become progressively occupied, leading to a faster reaction rate. However, this trend reaches a plateau—the saturation point—where all enzyme active sites are occupied. Further increases in substrate concentration will not lead to a significant increase in the reaction rate. It’s like filling seats in a stadium; eventually, there are no empty seats left.
Temperature: The Goldilocks Effect
Enzymes have an optimal temperature range at which they function most effectively. At lower temperatures, enzyme activity is reduced due to slower molecular motion. At higher temperatures, enzyme activity initially increases due to increased molecular collisions. However, excessively high temperatures can denature the enzyme, causing it to lose its three-dimensional structure and catalytic activity. This is analogous to cooking an egg: moderate heat cooks it well, but high heat makes it hard.
pH: The Acid-Base Balance
Similar to temperature, enzymes have an optimal pH range. Deviations from this optimal pH can alter the charge distribution of the amino acid residues in the active site, affecting substrate binding and catalytic activity. Extreme pH values can also denature the enzyme. This demonstrates that maintaining the correct pH is critical for optimal enzyme functionality. It's like maintaining a balanced ecosystem: a slight shift can disrupt the equilibrium.
Inhibitors: Blocking Enzyme Action
Enzyme inhibitors are molecules that reduce or eliminate enzyme activity. They can be competitive inhibitors, which compete with the substrate for binding to the active site, or non-competitive inhibitors, which bind to a different site on the enzyme, altering its shape and reducing its catalytic efficiency. Inhibitors play important roles in regulating metabolic pathways and are also used in drug design to target specific enzymes involved in disease processes. It's similar to a traffic jam; one car halting the traffic, or a separate issue causing congestion.
Activators: Boosting Enzyme Performance
Conversely, some molecules can enhance enzyme activity, acting as activators. These can bind to the enzyme, stabilizing the active site or inducing conformational changes that increase the enzyme's catalytic efficiency. This helps finely tune the rate of biochemical reactions. Imagine it as a helpful co-worker boosting the overall output.
Enzyme Classification: Six Major Classes
Enzymes are categorized into six major classes based on the type of reaction they catalyze:
-
Oxidoreductases: Catalyze oxidation-reduction reactions (transfer of electrons).
-
Transferases: Catalyze the transfer of functional groups (e.g., methyl, phosphate groups).
-
Hydrolases: Catalyze hydrolysis reactions (cleavage of bonds by adding water).
-
Lyases: Catalyze the addition or removal of groups to form double bonds.
-
Isomerases: Catalyze the rearrangement of atoms within a molecule (isomerization).
-
Ligases: Catalyze the joining of two molecules, often coupled to ATP hydrolysis.
The Significance of Enzymes in Biological Systems
Enzymes are indispensable for all forms of life. They are the driving force behind countless biochemical processes that sustain life, including:
-
Digestion: Digestive enzymes break down complex food molecules into smaller, absorbable units.
-
Respiration: Enzymes facilitate the stepwise oxidation of glucose to produce ATP (energy currency of the cell).
-
DNA Replication and Repair: Enzymes are crucial for duplicating and repairing DNA, ensuring genetic stability.
-
Protein Synthesis: Enzymes catalyze the formation of peptide bonds during protein synthesis.
-
Muscle Contraction: Enzymes regulate the processes involved in muscle contraction and relaxation.
-
Neurotransmission: Enzymes synthesize, release, and degrade neurotransmitters, enabling communication between nerve cells.
-
Immune Response: Enzymes participate in various aspects of the immune response, including the activation and inactivation of immune cells.
Enzymes in Industrial Applications and Medicine
The remarkable catalytic properties of enzymes have led to their widespread use in various industrial and medical applications:
-
Food Industry: Enzymes are used in baking, brewing, cheese making, and juice production to improve flavor, texture, and shelf life.
-
Biotechnology: Enzymes are used in the production of various biofuels, pharmaceuticals, and industrial chemicals.
-
Medical Diagnostics: Enzymes are employed in diagnostic tests to detect various diseases, such as heart disease and liver damage.
-
Medical Treatments: Enzymes are used in therapeutics to treat certain diseases, such as hemophilia and cystic fibrosis. Enzyme replacement therapy replaces missing or deficient enzymes.
-
Environmental Applications: Enzymes are used in bioremediation to clean up pollutants and toxic substances.
Ongoing Research and Future Directions
Research on enzymes continues to expand our understanding of their intricate mechanisms and their potential applications. Current research focuses on:
-
Enzyme Engineering: Designing and modifying enzymes to improve their catalytic activity, specificity, and stability.
-
Discovery of Novel Enzymes: Identifying and characterizing new enzymes with unique catalytic properties.
-
Understanding Enzyme Regulation: Elucidating the mechanisms that control enzyme activity in cells.
-
Development of Enzyme-Based Therapeutics: Creating new enzyme-based drugs and therapies for various diseases.
Conclusion: The Unsung Heroes of Life
Enzymes are the unsung heroes of biological systems, silently orchestrating the myriad chemical reactions essential for life. Their remarkable catalytic abilities, specificity, and susceptibility to regulation make them indispensable components of all living organisms and powerful tools for various applications. Continuing research promises to further reveal the intricacies of these remarkable molecules and unlock their full potential to benefit humankind. The understanding of enzyme catalysis is an ongoing journey of discovery, pushing the boundaries of our knowledge and impacting numerous fields of science and technology. From the smallest cellular processes to large-scale industrial applications, enzymes continue to amaze and inspire.
Latest Posts
Latest Posts
-
Chemicals That Absorb Light Are Called
Apr 11, 2025
-
What Property Is 8 8 0
Apr 11, 2025
-
4y 7 2y 3 Y 1 1
Apr 11, 2025
-
What Are The Functions Of Fruit
Apr 11, 2025
-
Which Of The Following Is A Proportion
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about Enzymes Are Catalysts That Increase The Rate Of Reactions By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.