Enter The Ions Present In A Solution Of Na2co3
News Leon
Mar 31, 2025 · 6 min read
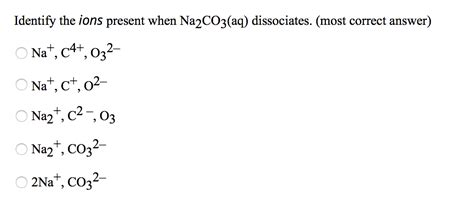
Table of Contents
Enter the Ions Present in a Solution of Na₂CO₃: A Deep Dive into Dissociation and Electrolytes
Sodium carbonate (Na₂CO₃), also known as washing soda, is a common inorganic compound with a wide range of applications, from water softening to glassmaking. Understanding its behavior in solution, specifically the ions it produces upon dissolving, is crucial for various chemical and industrial processes. This article delves into the intricacies of Na₂CO₃ dissociation, exploring the ions present, their concentrations, and the impact on solution properties.
The Dissociation of Sodium Carbonate
When sodium carbonate is dissolved in water, it undergoes complete dissociation, meaning it breaks apart completely into its constituent ions. This is because Na₂CO₃ is a strong electrolyte, a substance that readily ionizes in solution, producing a highly conductive solution. The dissociation process can be represented by the following equation:
Na₂CO₃(s) → 2Na⁺(aq) + CO₃²⁻(aq)
This equation shows that one formula unit of Na₂CO₃ produces two sodium ions (Na⁺) and one carbonate ion (CO₃²⁻) in aqueous solution. The "(s)" denotes the solid state of the sodium carbonate, while "(aq)" indicates that the ions are dissolved in water.
Understanding the Ions
Let's examine the ions produced in detail:
-
Sodium Ion (Na⁺): This is a monovalent cation, carrying a single positive charge. Sodium ions are relatively unreactive in aqueous solution and play a significant role in various biological processes. They are abundant in nature and are readily available.
-
Carbonate Ion (CO₃²⁻): This is a divalent anion, carrying two negative charges. The carbonate ion is a crucial component in many chemical reactions and is a relatively strong base. It can react with acids to form carbonic acid (H₂CO₃), which then readily decomposes into water and carbon dioxide. This reaction is responsible for the fizzing observed when an acid is added to a sodium carbonate solution:
CO₃²⁻(aq) + 2H⁺(aq) → H₂CO₃(aq) → H₂O(l) + CO₂(g)
Factors Affecting Ion Concentration
While the stoichiometry of the dissociation reaction (above) indicates a 2:1 ratio of Na⁺ to CO₃²⁻, several factors can influence the actual concentrations of these ions in a solution:
-
Concentration of Na₂CO₃: The initial concentration of dissolved Na₂CO₃ directly determines the concentration of its constituent ions. A higher concentration of Na₂CO₃ will result in higher concentrations of Na⁺ and CO₃²⁻. This relationship is directly proportional, assuming complete dissociation.
-
Temperature: Temperature influences the solubility of Na₂CO₃. Generally, the solubility of many salts increases with temperature. Higher temperatures can lead to a greater amount of Na₂CO₃ dissolving, thereby increasing the concentration of its ions.
-
Presence of Common Ions: The presence of other sodium salts or carbonate salts in the solution can affect the solubility and thus the concentration of Na⁺ and CO₃²⁻. This effect, known as the common ion effect, reduces the solubility of Na₂CO₃, lowering the concentrations of its ions.
-
pH of the Solution: The pH of the solution plays a critical role, particularly concerning the carbonate ion. In acidic solutions, the carbonate ion reacts with protons (H⁺), leading to the formation of bicarbonate (HCO₃⁻) and ultimately carbonic acid (H₂CO₃), which decomposes into water and carbon dioxide. This reaction reduces the concentration of free CO₃²⁻ ions in the solution. Conversely, in alkaline solutions, the concentration of CO₃²⁻ is largely unaffected.
Implications of Ion Presence
The presence of Na⁺ and CO₃²⁻ ions in a sodium carbonate solution has several important implications:
Conductivity:
The high concentration of ions in the solution makes it an excellent conductor of electricity. This is because the freely moving ions can carry electrical charge through the solution. The conductivity of the solution is directly proportional to the ionic concentration, providing a means to measure the concentration of Na₂CO₃ indirectly.
pH of the Solution:
A solution of Na₂CO₃ is alkaline, meaning it has a pH greater than 7. This is due to the basic nature of the carbonate ion, which can accept protons from water, leading to the formation of hydroxide ions (OH⁻):
CO₃²⁻(aq) + H₂O(l) ⇌ HCO₃⁻(aq) + OH⁻(aq)
The presence of hydroxide ions increases the concentration of OH⁻, increasing the pH. The extent of pH increase depends on the concentration of Na₂CO₃.
Reactions with Other Substances:
The presence of Na⁺ and CO₃²⁻ ions influences the reactivity of the solution with other substances. The sodium ion is generally unreactive, but the carbonate ion participates in many chemical reactions, as previously discussed. These reactions can include acid-base reactions, precipitation reactions, and complexation reactions.
Applications Leveraging Ion Properties
The properties of the ions produced by dissolving Na₂CO₃ are exploited in many applications:
-
Water Softening: The carbonate ion reacts with calcium and magnesium ions (which cause water hardness) to form insoluble precipitates, effectively removing these ions and softening the water.
-
Glass Manufacturing: Sodium carbonate is a crucial component in the production of glass, acting as a flux to lower the melting point of silica (SiO₂). The sodium ions contribute to the glass structure.
-
Detergents and Cleaning Agents: Its alkalinity and ability to react with acidic substances makes it a useful ingredient in detergents and cleaning agents.
-
Food Industry: It's used as a food additive (E500) in small quantities as a pH regulator and raising agent.
Conclusion
The dissociation of Na₂CO₃ in water yields a solution containing sodium ions (Na⁺) and carbonate ions (CO₃²⁻). The concentrations of these ions are influenced by various factors, including the initial concentration of Na₂CO₃, temperature, the presence of common ions, and the pH of the solution. The presence of these ions imparts specific properties to the solution, such as high conductivity and alkalinity, leading to its wide range of industrial and everyday applications. Understanding the behavior of these ions is crucial for leveraging the properties of sodium carbonate in diverse fields. Further investigation into the kinetics and thermodynamics of Na₂CO₃ dissolution can provide even more insights into its behavior in aqueous solutions. Exploring the interaction of these ions with other substances opens up a broad spectrum of possibilities for chemical reactions and applications, underscoring the fundamental importance of understanding the ionic constituents of this common compound. The interaction of Na₂CO₃ with other salts and complex systems warrants further research to fully elucidate its behavior in real-world scenarios. This detailed understanding allows for precise control over reaction pathways and optimizes the application of Na₂CO₃ in various industrial and scientific contexts. The versatile nature of this compound, stemming directly from its ionic components, establishes its enduring significance in chemistry and technology.
Latest Posts
Latest Posts
-
Where Is Most Of The Mass Of An Atom Concentrated
Apr 01, 2025
-
What Bone Articulates With The Acetabulum
Apr 01, 2025
-
What Is The Value Of Log Subscript 27 Baseline 9
Apr 01, 2025
-
How Many Chromosomes In Liver Cells
Apr 01, 2025
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Enter The Ions Present In A Solution Of Na2co3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
